Diagnostic &
Life Science
Product Services
Expertise and Resources to Achieve Commercialization
Projects Completed
Patents Filed
Years Experience
YOU’RE IN GOOD COMPANY























Case Studies
What We Do
Common Project Types Across the Continuum of Diagnostic Commercialization

Point-of-Care Instruments

Cartridge Design & Development
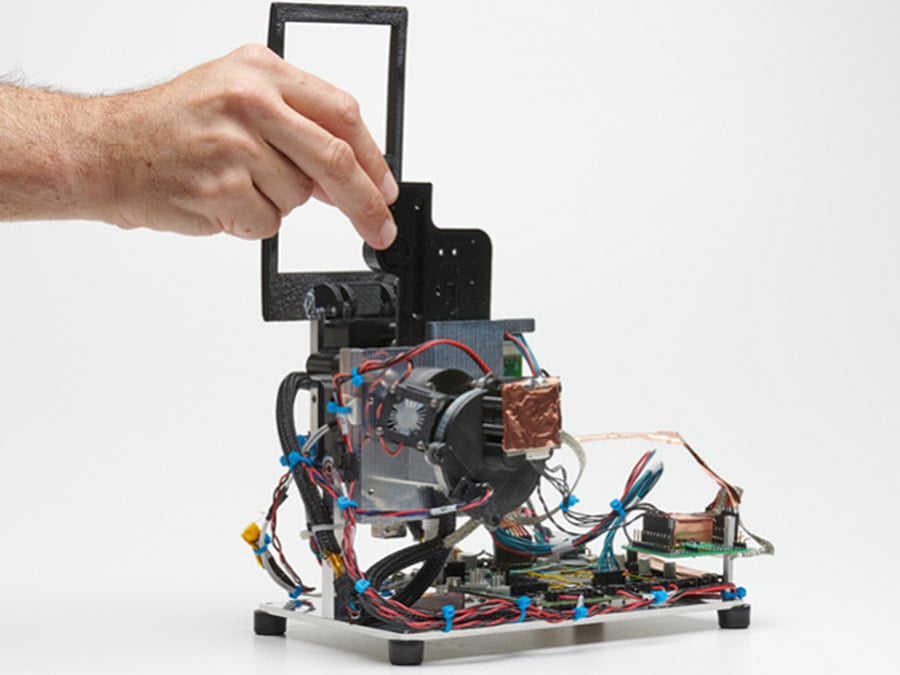
Pre-Built Technology & Proof of Concept Prototyping
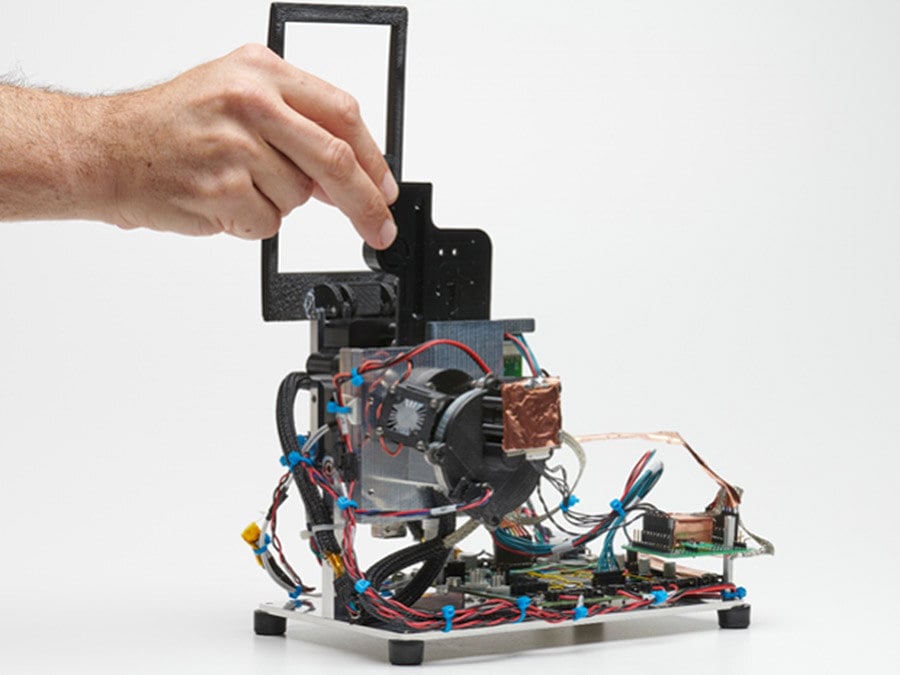
Centralized Lab Equipment Development

Commercial Roadmapping & Health Economics Modeling
Regulatory Pathway Strategy, Assessment & Submission
Controlling Risk
From Concept Through Manufacturing
We specialize in the design, engineering, and manufacturing of diagnostic technologies. Our team has experience with a multitude of platforms, including point-of-care, centralized lab equipment, cartridge development, as well as early-stage technology development and proof-of-concept prototyping with our pre-built technology stack.
Learn More →Human-Centered Digital Experiences
Meaningful Digital Touchpoints & Data Collection
We have deep expertise in user experience and user interface design for diagnostic systems and companion mobile solutions. Our process is rooted in understanding user needs and enabling seamless data collection. We have experience with a multitude of cloud platforms allowing for scalable connectivity, data exchange, remote accessibility, and better learning.
Clinical & Economical Evidence
Understanding Reimbursement, Pricing, and Go-To-Market Strategies
We partner with clients to successfully navigate the complex reimbursement landscape of novel diagnostic platforms. Our relationships with payers and providers help our clients understand coverage and pricing considerations, ensuring go-to-market strategies are aligned and viable.
Global Regulatory and CRO Services
Establishing your Product Pathway
We are recognized for our well-established relationships with FDA, EMA and market authorization agencies worldwide. With our track record of successful diagnostic product submissions, we'll help you determine the required regulatory pathway — whether that be 510(k), PMA, or De Novo classifications. Our team of clinical research experts can help design and manage trials on a global scale.
Need an expert opinion?
Contact one of our experts to accelerate your innovation today.




