INFLAMMATIX
Sepsis Diagnostics at
the Point-of-Care
Bringing world-class diagnostics to patients with Inflammatix

Pictured is the TriVerity™ Acute Infection and Sepsis Test system which includes the Myrna™ Instrument and the TriVerity cartridge.
Veranex and Inflammatix partnered to conceive, design, and manufacture Inflammatix’s Myrna Instrument. The system is in development, is not for sale, and does not have marketing approval or clearance from regulatory authorities in any jurisdiction.
Services
Industry
The Challenge
Partnering with Inflammatix to design and manufacture their Myrna Instrument for running the Inflammatix pipeline of point-of-care diagnostic tests including the TriVerity Acute Infection and Sepsis Test.
The Solution
Veranex worked with Inflammatix in a close partnership over five years (since 2018) starting with concept exploration of the system. Veranex integrated tightly with the Inflammatix team providing expertise in human factors and usability, electrical and firmware design, mechanical and manufacturing engineering, program management, design assurance and transfer to manufacturing.
“Working with Veranex was like working with an extended version of our own team. Tight integration amongst different disciplines at Veranex and Inflammatix was key to our success, and we always felt like partners, not clients.”
Paul Fleming
VP of Engineering
What is Sepsis?
Sepsis occurs when a patient has an infection (i.e. bacterial, viral) and a dysregulated immune response causing organ dysfunction.1 These infections are sometimes bloodstream infections but often occur locally (e.g. pulmonary, abdominal, skin, urinary tract) but with a sufficiently maladaptive and aggressive immune response to cause significant morbidity and mortality. 2 1.7 million patients a year are diagnosed with sepsis in the US alone, and it results in 350K deaths annually.3 Globally, 50 million patients are diagnosed with sepsis and 11 million die from it annually.4 Sepsis is also resource intensive and expensive. At $62 billion in annual spend, it’s Medicare’s most expensive hospital condition.5

Current Challenges in Diagnosing Sepsis
Proper sepsis diagnosis requires confirmation of infection and disease severity progression.6 Considering that earlier diagnosis is associated with better outcomes, obtaining these answers quickly is important.7 Unfortunately, there isn’t a single or collective set of tools that informs on acute infection and sepsis presence sufficiently sensitive, specific or fast enough.8
The Promise of the TriVerity Acute Infection and Sepsis Test
The Triverity Test is designed to inform on the likelihood of a bacterial infection, the separate likelihood of a viral infection on disease severity in about 30 minutes.9 These insights are intended to help physicians assess the presence, type and severity of infection, regardless where the source is located.10,11 Armed with this information, physicians may be able to make more informed and timely treatment decisions, including whether to prescribe antibiotics, whether additional tests should be ordered and what level of care is necessary (e.g. admit or discharge).12
References
1Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP)
2Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP)
3Rhee C, et al. “Incidence and Trends of Sepsis in US Hospitals Using Clinical vs Claims Data, 2009-2014.” JAMA. 2017;318(13):1241-1249.
4Rudd KE, et al., regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020 Jan 18;395(10219):200-211.
5Buchman TG, et al. Sepsis Among Medicare Beneficiaries: 3. The Methods, Models, and Forecasts of Sepsis, 2012-2018. Crit Care Med. 2020 Mar;48(3):302-318.
6 Source: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP)
7 Kim HI, et al., Sepsis: Early Recognition and Optimized Treatment. Tuberc Respir Dis (Seoul). 2019 Jan;82(1):6-14.
8 Roger PM, et al., Diagnostic uncertainty in infectious diseases: Advocacy for a nosological framework. Infect Dis Now. 2023 Sep;53(6):104751.
9 He YD, et al., The Optimization and Biological Significance of a 29-Host-Immune-mRNA Panel for the Diagnosis of Acute Infections and Sepsis. J. Pers. Med. 2021, 11, 735.
Project Phase 0
Research, Product Strategy, & Concept Generation
Our teams worked with Inflammatix to research user needs and use environments to support both sepsis and fever diagnostic applications. Concept areas were identified and developed to determine the best technologies to leverage an optimized system design solving for throughput tests, space available in different use environments, and user interactions with the capital system. Aspirational industrial design of the system was developed as a launching point for phase 1 activities.
Project Phase 1
Concept Development & Design Input
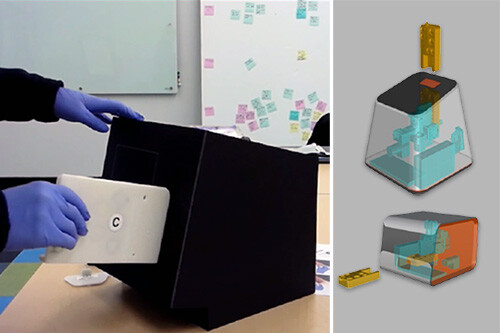
We worked with Inflammatix to conceive, design, and assemble feasibility breadboards and prototypes to de-risk the development pathway enabling them to do advanced assay testing. Our Human Factors team conducted user research, context of use, and use workflow analysis to ensure the product design was properly informed. The Industrial Design team used this groundwork to finalize the design intent to deliver a non-functional appearance model of the system for heuristic review.
Project Phase 2
Design Development & Design Outputs
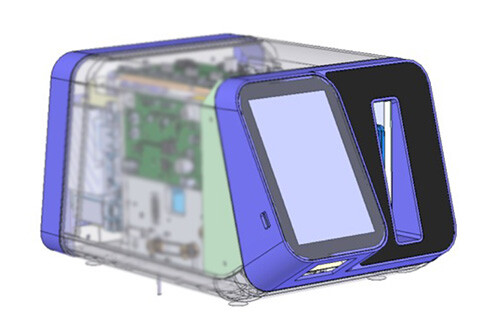
We focused on combining the learnings from phase 1 into an integrated instrument and cartridge for engineering verification testing. Leveraging our experienced engineering team, we provided detailed design, DFM, packaging prototypes, and supply chain assessment to Inflammatix. We also developed manufacturing processes and designed fixtures for assembly and test methods for in-process testing. Finally, we completed an engineering verification build of 10 units and tested to evaluate performance to the requirements developed in phase 1.
Project Phase 3
Design Verification
Our Manufacturing team built fully traceable instruments to be used for design verification testing, Engineering completed design verification testing including ASTM transportation testing on the Myrna Instrument to verify the packaging design, 61010 electrical safety and EMC testing on the Myrna Instrument, and firmware verification testing. Finally, the Supply Chain team assessed the Bill of Materials and placed orders for long-lead components to reduce supply chain and overall programmatic risk in future phases.
.jpg?width=500&height=333&name=ifx%20(1).jpg)
Project Phase 4
Process & Design Validation, Manufacturing/Design Transfer
Our engineering and manufacturing teams worked closely with Inflammatix to build and release over 20 Myrna Instruments for use in clinical studies and our Human Factors team is executing a summative study. Design and manufacturing files were transferred to support commercial build scale-up.


Working Together from Concept to Manufacturing
A close working relationship between the Veranex and Inflammatix teams has been critical to achieving long term success in this program. We’ve continued to be flexible with support through all phases, effectively working as an extension of the client’s technical team and fully integrating ourselves with the client’s Consumable, Software, and Instrument Design teams.
Veranex completed the work by delivering Myrna Instruments for clinical studies as well as transfering knowhow and equipment to Inflammatix for the eventual start of commercial builds.
Inflammatix, Inc., headquartered in Sunnyvale, California, designs, develops, and manufactures diagnostic tests. Inflammatix was founded in 2016 based on its team’s expertise in interpreting the immune system’s response to disease. Inflammatix is building a deep pipeline of diagnostic tests to run on its proprietary Myrna Instrument.
Inflammatix Receives FDA Clearance for First-in-Class TriVerity™ Test
Need an expert opinion?
Contact one of our experts to accelerate your innovation today.

