According to the most recent draft of the International Council on Harmonisation (ICH) Guideline for Good Clinical Practice (GCP) E6(R3), “The aim of monitoring is to ensure the participants’ rights, safety and well-being and the reliability of trial results as the trial progresses.” As such, monitoring activities play a key role in the trial’s quality management strategy and ensuring trial and data integrity.
Risk-based management (RBM) is pivotal to quality management and involves risk identification, evaluation, control, communication, review, and reporting. To achieve this, clinical trials benefit from both site monitoring and centralized monitoring, depending on the strategy and trial design.
Centralized monitoring
Centralized monitoring can be an efficient way for central monitors (CMs) to use the study data to identify sites or processes that pose risks and require remediation. Once the risks are identified, their evaluation allows CMs to identify operational risk factors and the potential impacts. Surfacing and communicating these risk factors allow the CM to either eliminate or mitigate and control the risks effectively. A functional central monitoring process should include a brief analysis with observations and mitigation steps to be taken, and consultation with different trial stakeholders contributes to the accountability within the central monitoring process.
Central monitoring reports include the site-specific risk factors, acceptable threshold levels, and how mitigation actions improved the site performance. Although reporting frequency may vary throughout the trial depending on the availability of data, “reports of…centralized monitoring should be provided to the appropriate sponsor staff…in a timely manner for review and follow-up,” according to ICH E6(R3).
Advantages of a CRREG platform
A centralized risk reporting, escalation, and governance (CRREG) platform offers a relatively easy and user-friendly method for CMs to manage centralized monitoring of trials. Purpose-built for centralized monitoring, it helps oversee risk identification, evaluation, and reporting and automates time-consuming standardized processes, such as Study Insight Reports (SIRs) and Monitor Insight Reports (MIRs).
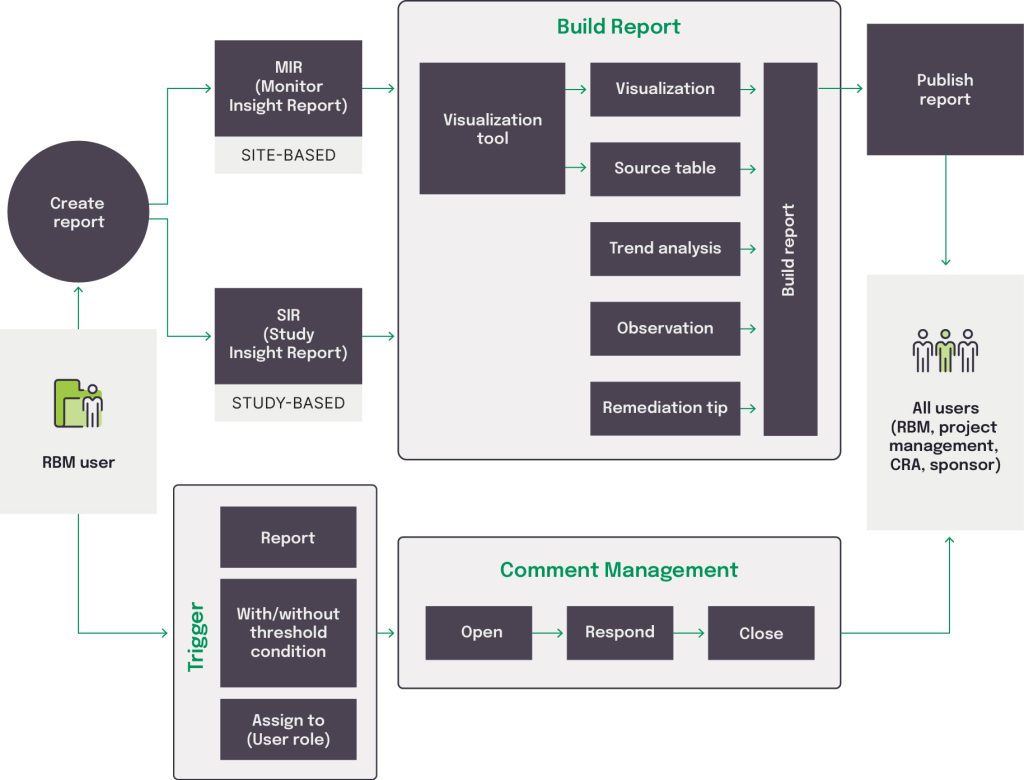
Adhering to the centralized monitoring plan, a CRREG helps with the early detection of risk for the CM when manual or automated triggers indicate that a predefined threshold is reached. Using prescriptive analysis, the platform derives an overall risk score using predefined key risk and performance indicators, which then drives the extent, timing, and frequency of monitoring visits. The risk scores guide the monitoring schedule as well as specific actions to be taken during site visits.
Finally, an audit trail should always be made available so that teams can review actions retrospectively to improve future CRREG platform implementations.
These and other features are available in the Veranex CRREG platform, which we developed based on our years of supporting clients with their centralized monitoring needs. Contact one of our Data Management & Analytics team members to discuss how it could support your future studies.
