We help medtech leaders ↓
Achieve preclinical success. Our team of experts specialize in GLP and Non-GLP research and have extensive experience across many therapeutic areas.

OUR EXPERIENCE, YOUR SUCCESS
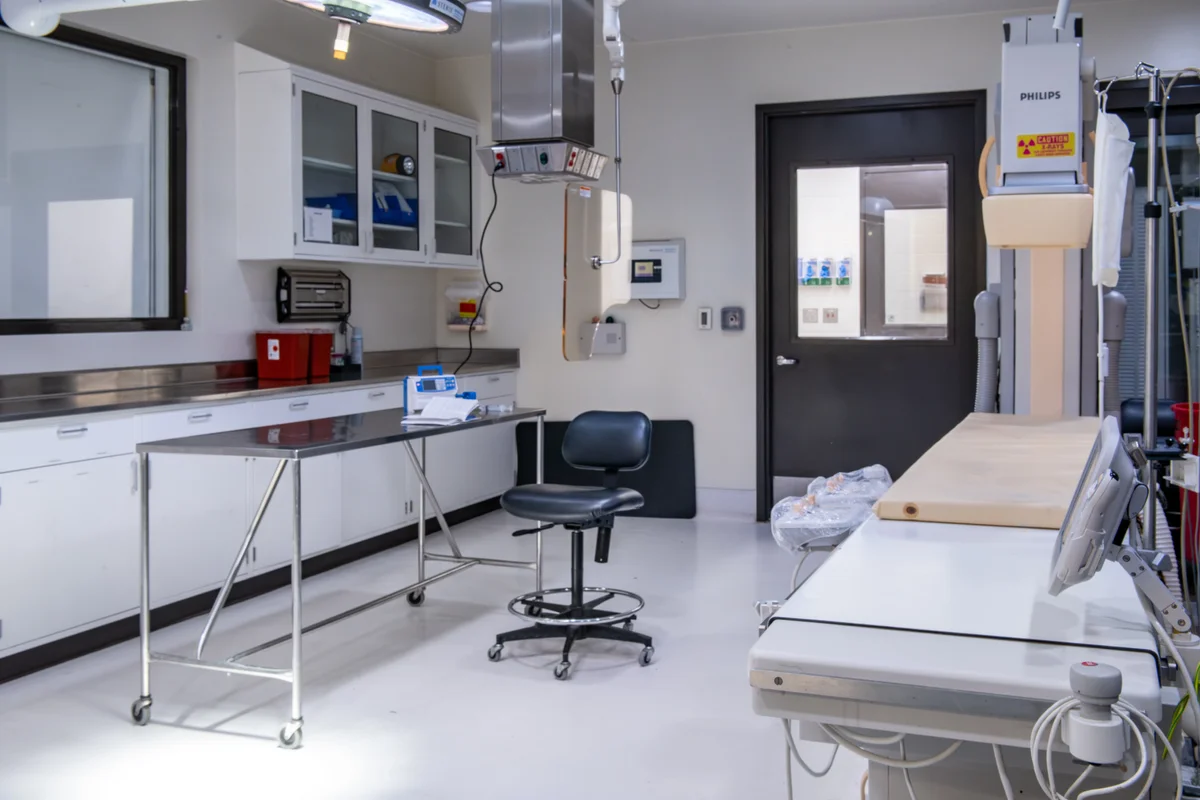
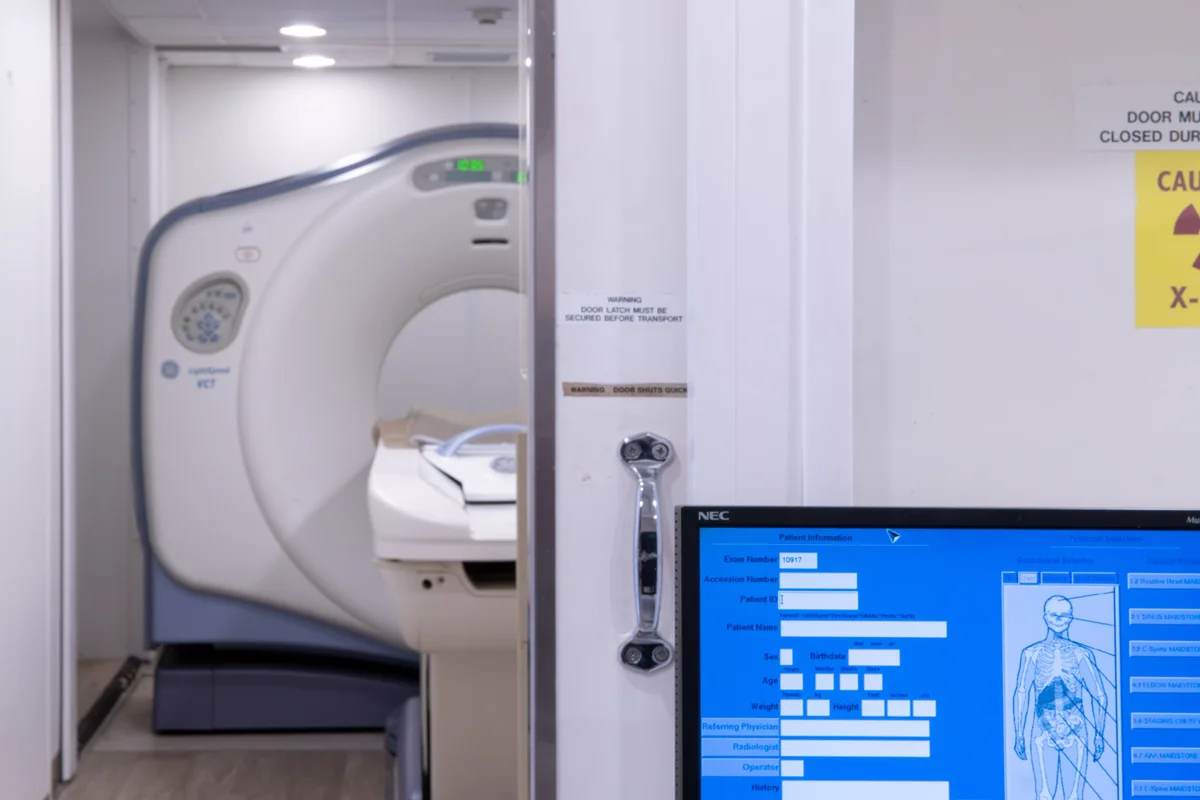
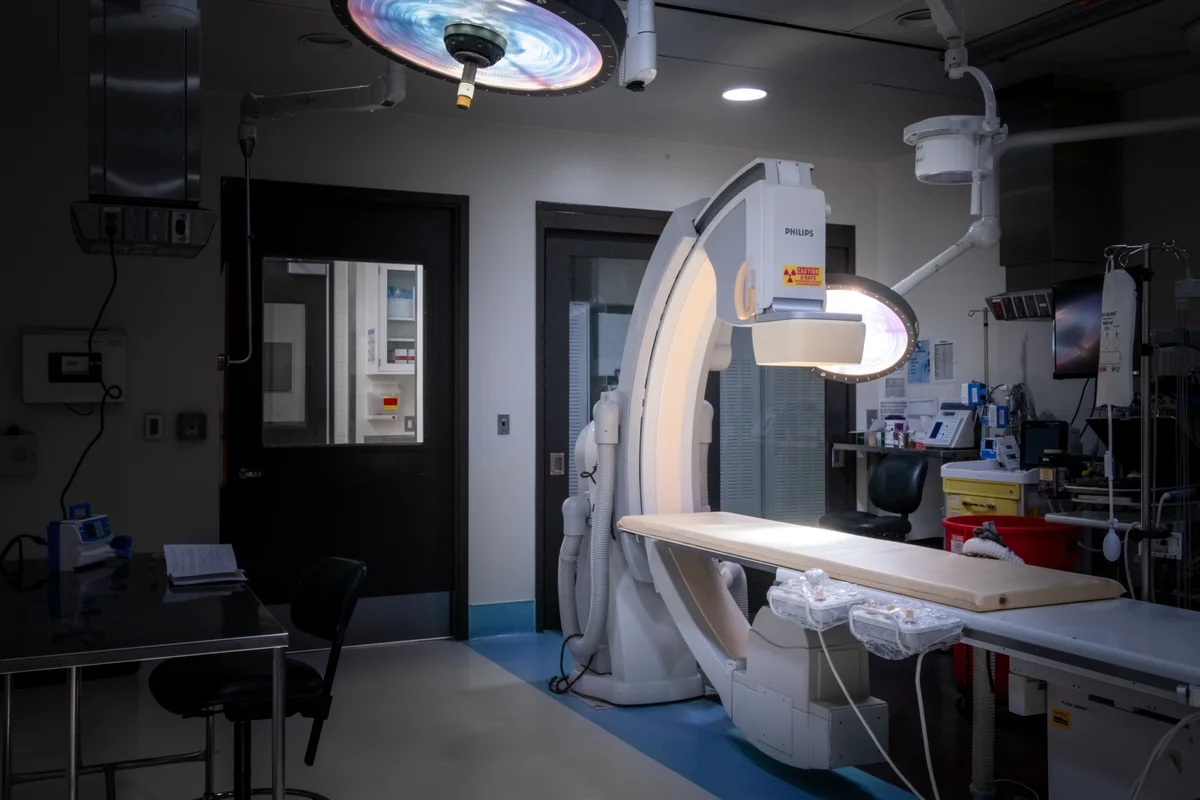
In-House Pathology Labs
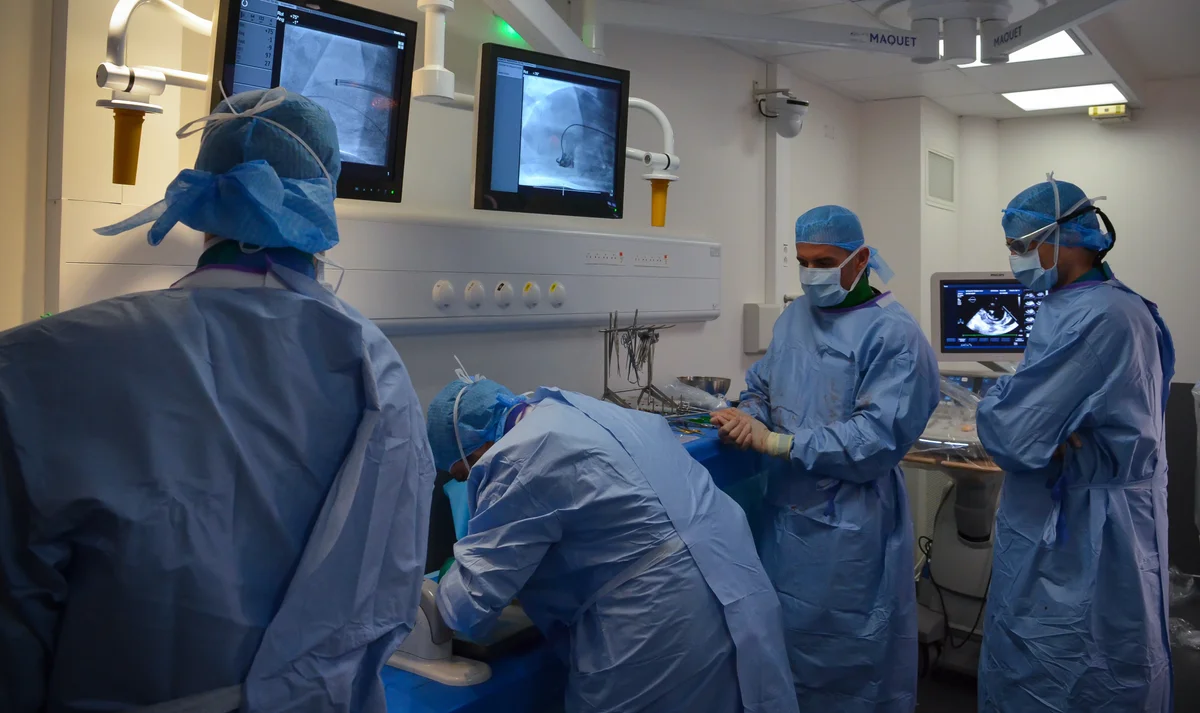
Operating Rooms
Therapeutic Areas Served
Full Time Veterinarians
Years of Experience in Structural Heart and Cardiovascular
Submissions, no questions asked
HOW WE HELP

Therapeutic Expertise
Cardiovascular
Vascular Intervention
Neurology
Gastroenterology
Orthopedics
Regenerative and Wound Healing
Your Title Goes Here
Your Title Goes Here
Your Title Goes Here
Your Title Goes Here
Your Title Goes Here
Orthopedics
Experts in studies focused on a broad range of orthopedic procedures
Experience includes:
- Tendon Repair
- Bone Repair
- Tendon Replacement
- Bone Segmentation
- Bone Fracture Healing
- Osseo Integration
- Spine Fusion
- Infection
- Degenerative Disk Disease
- Prosthetics
- Amputation
- Osteochondral Defect
Your Title Goes Here
INSIGHTS
Building Blocks of Quality: The Role of Design Control in Medical Device Development
Playing Defense: Cybersecurity for Diagnostic Devices
Protected: Expert Insights: Project Management with Matt Perry
Password Protected
To view this protected post, enter the password below: