
Prof. Alexandre Carpentier, Neurosurgeon at the Pitié-Salpêtrière University Hospital (AP-HP)
Prof. Alexandre Carpentier is a world-renowned neurosurgeon, researcher, university professor, and head of the department of neurosurgery at the Pitié-Salpêtrière University Hospital and the inventor of the groundbreaking medical device, the SonoCloud®. As a spin-off from the Assistance Publique – Hôpitaux de Paris (AP-HP) and Sorbonne University, Carthera® leverages the research work and inventions of Prof. Carpentier, internationally recognized for his expertise in applying new technologies to the brain. Carthera® has pioneered the SonoCloud®, an intracranial implant designed to temporarily open the Blood-Brain Barrier (BBB) and is revolutionizing the treatment of brain tumors. Let’s delve into the genesis of this ultrasound-based technology and explore the achievements of Carthera®.
We, at Veranex Preclinical Services, have been known historically to have been associated with the birth of many structural heart devices that have improved human healthcare.
The truth is, although cardiology is our strong suit, our expertise extends far beyond, encompassing fields such as vascular and neurovascular, urology and nephrology, fetal surgery, digestive surgery, orthopedics, and definitely neurology. Our studies have explored a wide range of topics, from vagus nerve to cortical and deep brain stimulation, as well as treatment and ablation of central nervous system tumors. Neurology is experiencing a notable surge in the development and utilization of advanced imaging, diagnostic, and therapeutic medical devices.
This progress brings forth a multitude of considerations, ranging from selecting the most appropriate animal model, establishing optimal anesthetic protocols, and implementing postoperative treatments, to conducting comprehensive imaging and histopathology analyses of device-body interactions. At our laboratory, it is ingrained in our DNA to provide unwavering support to our clients, accompanying them in the validation of their devices through a scientifically and technically rigorous approach. We stand by their side, offering expertise and collaboration every step of the way. In 2017, we had the privilege of collaborating with Professor Alexandre Carpentier and Carthera, which resulted in highly successful proof of concept, feasibility, and regulatory studies conducted within our lab. We had the opportunity to interview Alexandre Carpentier, who shared his remarkable journey as a surgeon, scientist, and entrepreneur, leading to groundbreaking advancements in human healthcare.

Olivier Chevènement, DVM, PhD
Veterinary Surgeon –
Scientific and Technical Manager –
Veranex’s neurology expert.
Welcome, Professor Carpentier. As a neurosurgeon, you have invented a revolutionary device for the treatment of brain tumors based on your observations during your practice. Could you tell us more about the institution that sparked the genesis of this ultrasound-based technology?
AC: Certainly. Through my practice as a neurosurgeon at the Pitié-Salpêtrière University Hospital, I quickly turned to medical imaging to operate on my patients with greater precision and came up with the idea of using a subcutaneous ultrasound emitter to close the surgical site. In fact, a neurosurgeon only sees the surface of their surgical field, which is not much, while there are numerous processes occurring beneath. I was convinced that MRI would be the best eye for future surgery, so I focused my research on minimally invasive therapies compatible with MRI. This led to the development of the SonoCloud® device in 2010, designed to open the BBB temporarily using low-intensity pulsed ultrasound. Following up on this discovery, Carthera® emerged as a spin-off from the AP-HP and Sorbonne University, with the goal of translating my research and invention into a tangible solution. In addition, since its inception, the technical and clinical development of the SonoCloud® has received great support from the French National Research Agency (ANR), the French public investment bank (Bpifrance), the European Innovation Council (EIC), and the National Institutes of Health (NIH) in the United States. There is a significant unmet need for new treatments for glioblastoma patients, and last year the FDA acknowledged the innovative potential of the
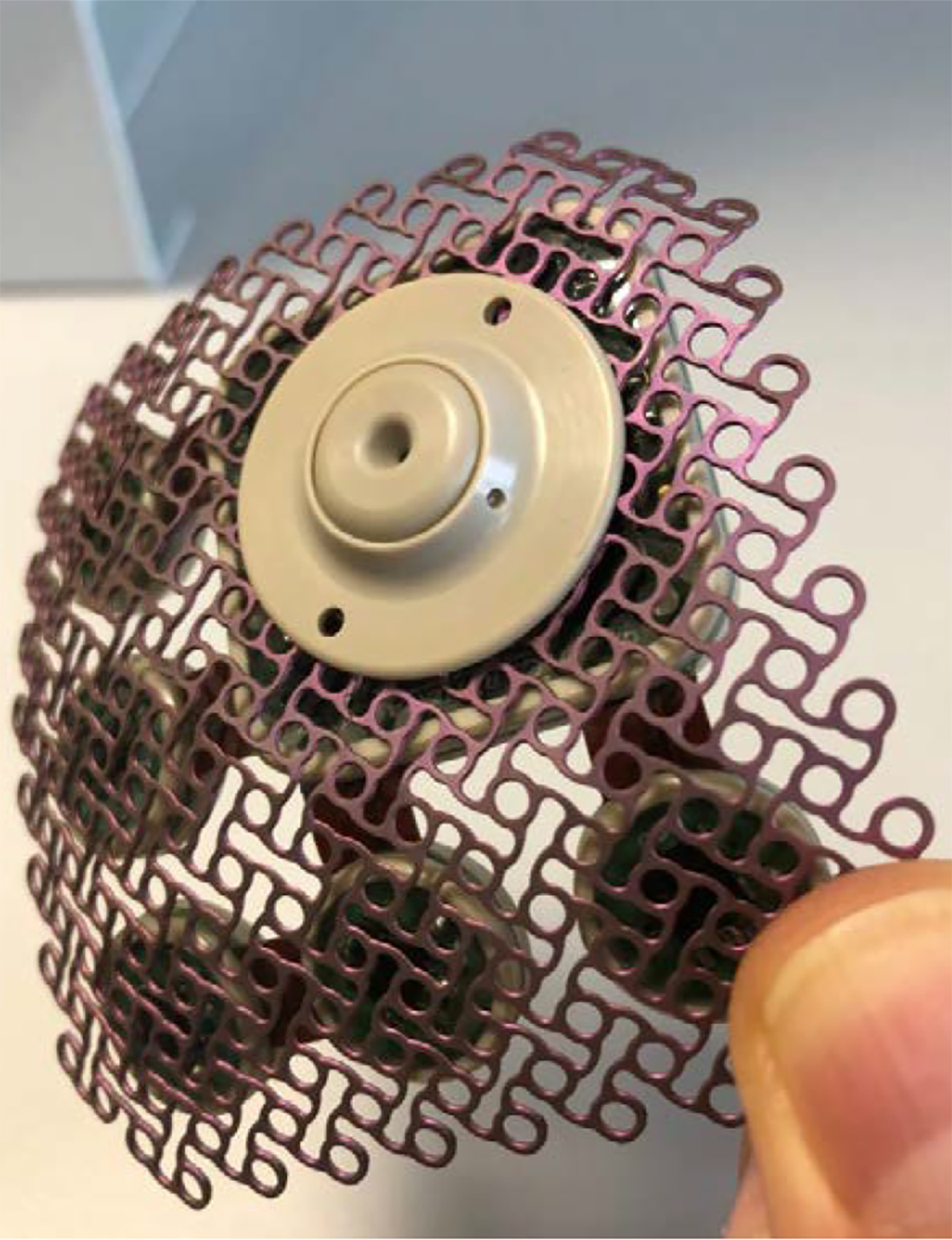
SonoCloud® device of Carthera®
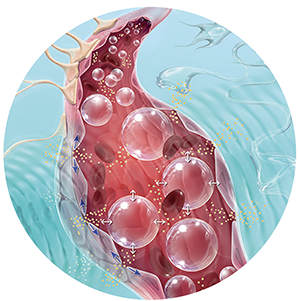
The SonoCloud® technology relies on using low intensity ultrasound in combination with intravenous injection of a microbubble agent to stimulate a therapeutic effect.
Sonocloud approach through the granting of this breakthrough designation. By harnessing the power of MRI and ultrasound, we can revolutionize the field of brain tumor treatment and deliver therapies with enhanced precision and efficacy, and we are making this assumption a reality in patients, as the device is undergoing clinical trials in Europe and the United States.
Could you tell us about the SonoCloud® technology and what it takes to bring such an innovative technology to patients?
AC: My work focuses on opening the BBB using ultrasound. One of the major challenges in neuroscience is that drugs, particularly chemotherapy, have difficulty entering the brain parenchyma due to the tightness of the blood vessels. Indeed, the BBB is a vascular endothelium composed of a triple barrier that prevents entry into the brain parenchyma of all molecules but small lipophilic molecules that do not contain more than 8 hydrogen bonds and correspond to 2% of the total of molecules. While this barrier protects us, it makes it difficult for medications to penetrate in cases of illness, and only 5% of drugs developed for the nervous system can penetrate and exert a therapeutic effect. As a result, patients with malignant brain tumors have a very short life expectancy.
It has been known for a long time that ultrasound has the ability to induce mechanical vibration in blood vessels, thus opening the BBB. However, the intensity of ultrasound must be significant, and its toxicity prevents its application in humans. In 2001, the idea emerged to transmit low-intensity pulsed ultrasound that would no longer interact directly with the brain tissue but would instead cause microbubbles present in the cerebral vasculature to vibrate. These microbubbles are previously injected into the general circulation through intravenous administration.
Therefore, by emitting low-intensity pulsed ultrasound in the brain at the resonant frequency of these microbubbles, they will vibrate (cavitation) when they pass through the ultrasound field. This vibration of microbubbles induces mechanical stress on the vessel wall. Only two minutes of ultrasound emission and vibration of these microbubbles are sufficient to open the BBB for one hour. Importantly, this process is completely non-traumatic and reversible due to the low intensity of the emitted ultrasound.
It is in 2010 that the full potential of MRI was harnessed through the realization of closed-skull interventions using an intratumoral laser probe introduced via stereotaxy under MRI guidance. In 2012, thanks to the work of physicist Mathias Fink from ESPCI in Paris, ultrasound made its entry into neurosurgical therapies. The missing piece was the concept of «time reversal.» Ultrasound waves are heavily attenuated and defocused by the skull, making them uncontrollable. With time reversal, aberrations can be corrected, as confirmed by MRI.
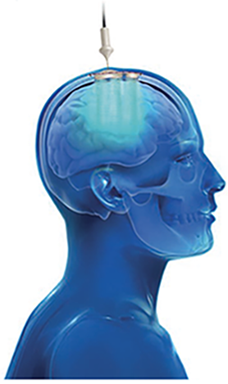
The SonoCloud® is a minimally invasive device implanted in a skull window, below the skin, and is invisible. The system is activated using a transdermal needle connection to an external control unit.
If ultrasound can be emitted from outside the skull, it can also be emitted through an implant installed under local anesthesia within the thickness of the bone. This is the SonoCloud®. This technique, which I have started developing at the Pitié Salpêtrière, allows for openings of the BBB to be achieved in a few minutes, bypassing the issues related to the bone and eliminating safety concerns and the need for MRI feedback. Since 2010, we have conducted a large series of preclinical studies in collaboration with research laboratories, public and private, including Veranex, which at that time was called IMMR. Your know-how and extensive expertise in preclinical science and large models in the field of neuromodulation helped us to evaluate our ultrasound-based technology prior to our successful transition to the clinic.
We saw in the Lancet Oncology the excellent results from your phase I clinical trial in glioblastoma patients – congratulations! We would love to hear your comments on how the groundbreaking results obtained with this trial using the SonoCloud-9 system open up new possibilities for glioblastoma patients and pave the way for future advancements in brain tumor treatments.
AC: The clinical results obtained by Carthera® earlier this year are truly exciting and promising for glioblastoma patients. The study has demonstrated the potential for safe and tolerable delivery of treatment using the SonoCloud-9 system. It is a groundbreaking achievement as it is the first study to quantifiably measure the impact of ultrasound-based BBB opening on chemotherapy concentrations in the human brain. The opening of the BBB resulted in a remarkable four- to six-fold increase in drug concentrations, as observed with two different chemotherapy drugs—paclitaxel and carboplatin. This advancement holds immense potential for improving outcomes in these patients.
The findings from this study, along with the positive outcomes observed in the Phase 1/2 trial using carboplatin – from which the FDA Breakthrough Device designation to Carthera was based – support our plan to advance to Phase 3 clinical trials. This trial will start later this year. The objective of this pivotal trial is to investigate whether the SonoCloud-9 system can significantly extend the survival of patients receiving this innovative treatment compared to the current standard of care.
The successful development of this technology is the result of medical multidisciplinarity involving neurosurgeons, neuroradiologists, and neuro-oncologists, as well as scientific multidisciplinarity involving physicians, physicists, chemists, electronic engineers, and computer scientists. It is through this collaboration and integration of various fields that groundbreaking and hybrid technologies have emerged, combining diagnostics and therapeutics, physics and biochemistry, surgery, and medicine.
Furthermore, it is important to note that while surgeries have traditionally relied on the surgeon’s expertise enhanced by microscopes or endoscopy, a significant portion of future surgical procedures may be guided solely by MRI. This advancement enables three-dimensional anatomical evaluation and real-time metabolic and functional assessment, reducing the need for extensive surgical incisions and allowing for procedures to be performed under local anesthesia on an outpatient basis. Undoubtedly, the next twenty-five years hold tremendous potential for unforeseen and remarkable progress in the field of brain treatments.
Thank you, Prof. Carpentier, for sharing your time and providing me with your insights.

© Veranex Preclinical Services 2023. All rights are reserved. Prepared with: Olivier Chevènement, Nicolas Borenstein, Cécile Jung Editor: Nicolas Borenstein – Photos: © Nicolas Borenstein Videos and previous newsletters on our services are available online here:
https://veranex.com/preclinical-services
www.veranex.com
