Introduction
Nanomaterials, tiny scientific wonders that are invisible to the naked eye, hold the potential to revolutionize numerous facets of our lives. Among these, medical devices stand as a promising frontier. The unique properties of nanomaterials, stemming from their small size and high surface-to-volume ratio, make them highly desirable in the field of medical devices.
Defining Nanomaterials
Before diving into the world of nanomaterials, we first need to define them. According to ISO/TR 10993-22, “a material is considered a nanomaterial when it has a size at the nanoscale including external and internal dimensions, i.e., when it has a size or is composed of structures with a length of approximately between 1 nm and 100 nm”. The EU Commission defines nanomaterials according to 2011/696/EU, updated in June 2022, as: “Nanomaterial” means a natural, incidental, or manufactured material consisting of solid particles that are present, either on their own or as identifiable constituent particles in aggregates or agglomerates, and when 50% or more of these particles in the number-based size distribution fulfil at least one of the following conditions:
- A: One or more external dimensions of the particle are in the size range 1 nm to 100 nm;
- B: The particle has an elongated shape, such as a rod, fibre or tube, where two external dimensions are smaller than 1 nm and the other dimension is larger than 100 nm;
- C: The particle has a plate-like shape, where one external dimension is smaller than 1 nm and the other dimensions are larger than 100 nm.
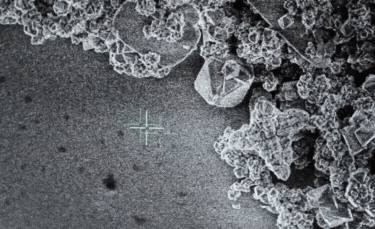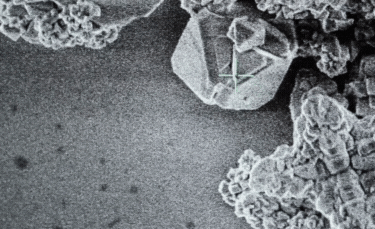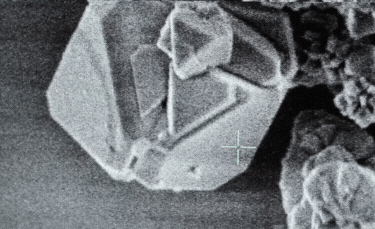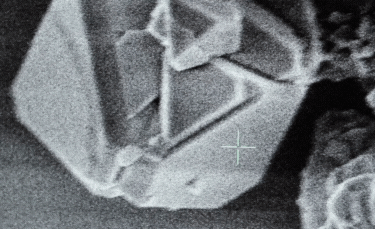
A: Particle size ranging from 1nm to 100nm. |

B: Fibre-shaped nanoparticle. |
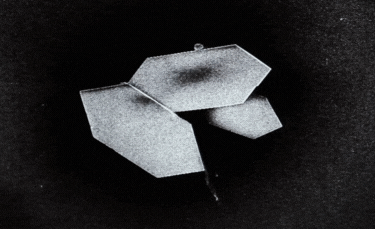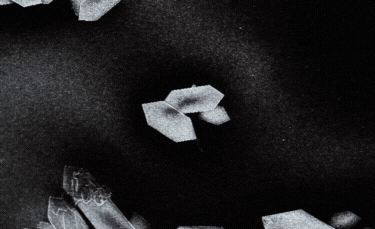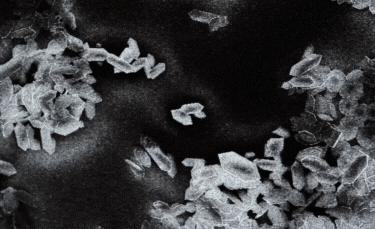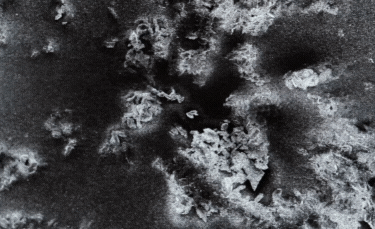
C: Plate-shaped nanoparticle. |
Biological Risks of Nanomaterials in Medical Devices
As presented above, nanomaterials, due to their small size and high surface area, present distinctive physico-chemical properties. However, these properties also entail unique biological risks. Their small size, falling within the same size range as structures at the subcellular level, and high reactivity enable them to readily enter biological systems, potentially leading to unexpected interactions and adverse biological responses. For example, nanomaterials can interact with proteins or enzymes, potentially altering their structures and disrupting their functions, thereby triggering adverse biological reactions.
Nano-structures or nano-coatings integrated into medical devices have been shown to influence cell alignment, morphology, and gene expression, while the potential of nanomaterials to form aggregates or agglomerates further complicates the risk landscape. This situation is further compounded by the fact that nanoparticles of identical chemical composition can exhibit varying toxicological risk profiles, depending on factors like shape, size, intended application, and route of exposure.
Diversity of Nanomaterials in Medical Devices
In today’s healthcare landscape, an array of innovative medical devices incorporating nanomaterials have emerged. These include antibacterial gowns, advanced wound dressings, dental cements with nano-diamond coatings, bone cements enriched with carbon nanotubes, and implants featuring state-of-the-art nano-surface coatings. Furthermore, the use of free iron oxide or gold nanomaterials as medical devices directly administered to patients exemplifies the expanding horizons of nanotechnology within healthcare.
At this point in time, it is important to eliminate one common misconception: patient exposure to nanomaterials from medical devices is not confined solely to those intentionally designed for controlled release. While medical devices containing “free” nanomaterials or nano-objects/nanostructures on their surface or within carry a higher exposure potential, nanomaterial exposure can also result from device degradation, wear, or mechanical processes like grinding or polishing.
Given this array of biological risks and exposure routes, an important question arises: how should we approach the assessment of biocompatibility for medical devices that either contain, generate, or are composed of nanomaterials?
Methodology for Biological Assessment of Nanomaterials in Medical Devices
ISO 10993-1:2018 provides a general framework for conducting biological risk assessment of medical devices, considering the device’s type and duration of its contact with the human body (Read more in our blog post about Biocompatibility of Medical Devices). This framework is known to be robust and flexible and, in general, is also applicable to devices that contain, generate, or are composed of nanomaterials, despite the distinctive properties of these materials compared to their conventional counterparts. ISO/TR 10993-22 serves as another valuable guidance document that supplements this framework, aiding in the safety evaluation of devices containing, generating, or composing nanomaterials.
Another relevant resource is the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) Opinion on “Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices”. This report suggests a phased approach based on the potential release and characteristics of nanomaterials to assess their risk in medical devices. The phases encompass particle release (phase 1), particle distribution and persistence (phase 2), hazard assessment (toxicological evaluations) (phase 3), and risk assessment/characterization (phase 4). For phase 1 assessment, Table 1 of ISO 10993-22 can be a handy reference. This table provides a list of five categories of nanomaterials that may be contained, present or generated by your medical devices. Click to watch our webinar, where this topic is covered in more detail.
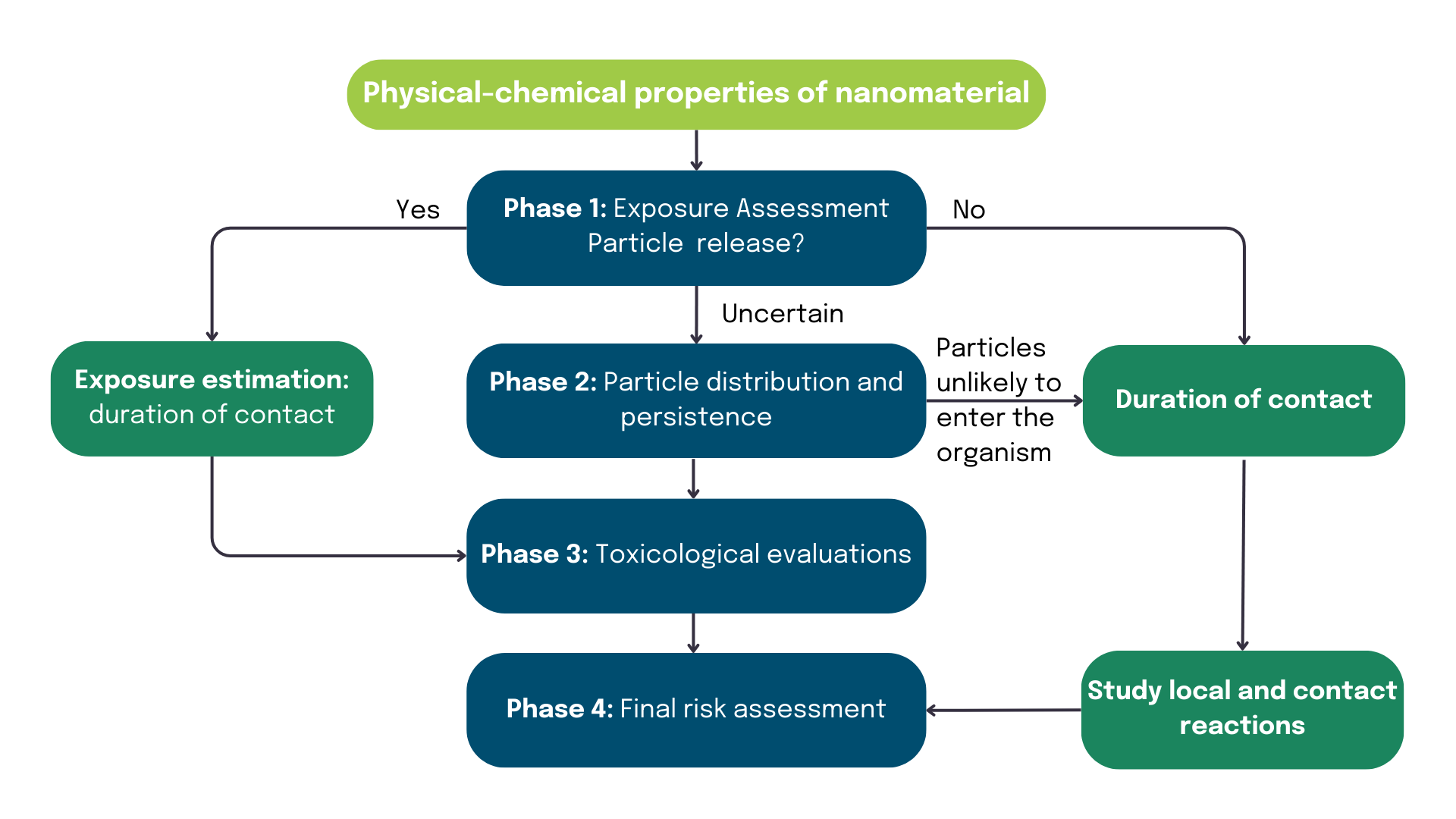
SCENIHR phased approach
It’s important to note that certain devices might fall into more than one category. In such cases, an evaluation should be conducted for each applicable category, taking into account the specific considerations provided under that category. These considerations encompass aspects such as characterization of physico-chemical properties, release kinetics, toxicokinetics, tissue distribution, and more. Regardless of the applicable category(ies), a common consideration is the evaluation of the physico-chemical characterization, which can be conducted following guidelines available in ISO/TR 13014:2021. Understanding the physico-chemical properties of the released nanomaterials is essential for establishing the nature of released particles, the rate of release, and the factors influencing this release. This step stands as a critical component of biological evaluation.
Subsequently, it is crucial to delve into potential exposure to nanomaterials in medical devices along with their persistence potential. This facet aims to determine the extent of nanomaterial exposure experienced by the patient and also considers whether the exposure makes the nano-objects accessible for local tissue responses and/or systemic exposure. Multiple factors must be considered for exposure assessment such as intensity, frequency, and duration of contact, route of exposure, intake or uptake rates, and bioavailability. An estimation of the potential external exposure to the nanomaterial and internal systemic exposure across all organ systems is discussed in SCENIHR Opinion and covered in detail in this Veranex webinar.
Following the exposure assessment, the focus shifts to identifying and characterizing the potential hazards arising from contact with nanomaterial. This hazard assessment involves selecting toxicity tests relevant to the nature of exposure and potential persistence in specific organs. The information gathered thus far serves as valuable input for final risk evaluation/characterization.
The estimated risks can then be compared to the risk associated with the use of comparable devices not incorporating nanomaterials. Ultimately, the potential benefit for the patient should be considered in the final benefit-risk evaluation.
Conclusion
As the industry ventures deeper into the nanoworld and explores the possibilities of nanomaterials in medical devices, the assessment of biocompatibility should also move forward at the same pace. Leveraging established frameworks like ISO 10993-1:2018 and guidance documents such as ISO/TR 10993-22 and the SCENIHR Opinion can pave the way to accurately assess the biological risks associated with the use of nanomaterials in medical devices. It is a journey that requires a meticulous understanding of nanomaterial properties, exposure pathways, and biological responses, with the final goal of enhancing patient safety.
How Can Veranex Support You?
Veranex has a dedicated team of nanomaterial and biocompatibility experts, operating as your partner in evaluating the possibility of your device either containing or generating nanomaterials. Our teams conduct biological risk assessments of such devices and define specific testing for nanomaterials. Combining regulatory and technical expertise, our experts pursue an efficient path to compliance, allowing you to keep your project moving while staying on budget. Contact us today to get started!
This article was written by Dr. Neeru Mittal and Dr. Adrien Marchand, biocompatibility experts at Veranex.

