Veranex is proud to share the latest issue of our Preclinical Insights publication. In this issue we talk with Dr. Laurence Fiette, Head of Veranex’s Pathology Department.

Dr. Laurence Fiette, Head of Veranex’s Pathology Department. With Veranex for 16 years, she shared insights about what sets our pathology department apart.
Preclinical assessments of medical device safety, efficacy, and performance rely on pathology evaluations to gather critical information about the biological responses to the device or the biomaterials used. Despite its importance, few organizations offering preclinical services have an
in-house pathology department. At Veranex, we believe
this is an important integration to provide our clients timely, cost-effective access to all the services and expertise they need from a single provider. As with everything at Veranex, we approached our in-house pathology services intentionally — with continued support and input from medical device and veterinary experts.
Laurence, could you give our readers a brief overview of the services that the preclinical pathology department provides for Veranex clients?
LF: In a nutshell, our team provides integrated, comprehensive services for preclinical medical device studies, including macroscopic evaluation, sample processing, histopathological evaluation, interpretation, and reporting. We are FDA and OECD GLP compliant for medical device preclinical studies and support companies that are evaluating their products across many therapeutic areas, including the following:
• Heart valves
• Endovascular and coronary stents
• Vascular conduits
• Orthopedic implants
• Artificial cartilage
• Surgical sealants
• Ophthalmic implants
• Artificial ureters
• Implantable patches
• Thrombectomy devices
• Deep nerve stimulators
With a sole focus on medical devices, our pathology team is particularly experienced in evaluating implantable devices (Class III or IV) with emphasis on structures of the heart and CV stents. Additionally, our experience with drug delivery devices supports neurobiological applications.
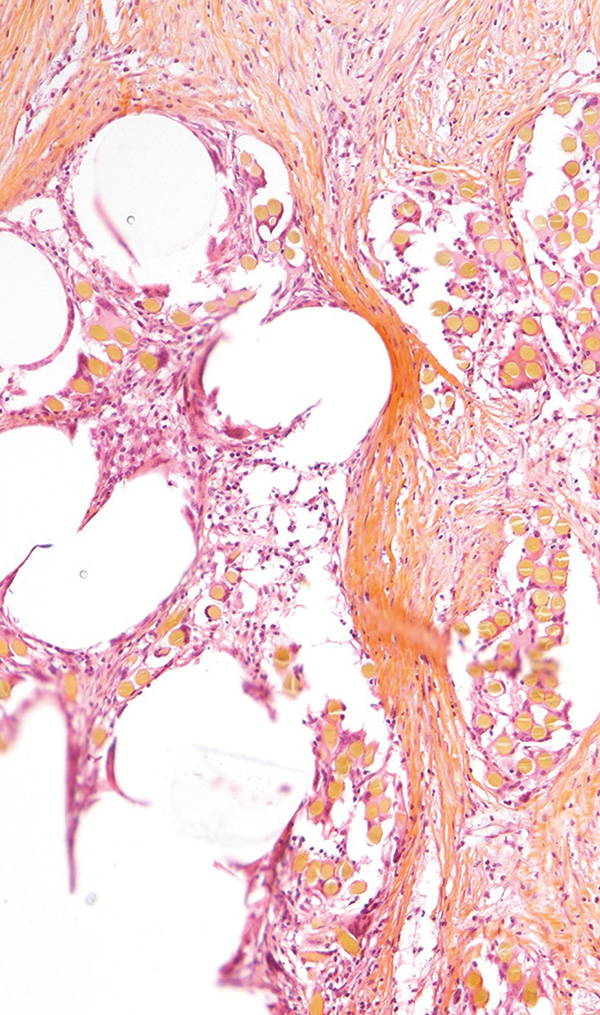
Porcine, Abdominal mesh, Paraffin section, HE&S stain
Leveraging our technical platform, we also offer resin sectioning services for medical devices and hard tissues, a highly specialized technique not commonly available in every histopathology laboratory. Our Faxitron Cabinet produces high-resolution radiography of explanted tissues and medical devices for assessment of device integrity and tissue or device calcification, and we can also access a micro-CT to generate high-resolution (~25 microns) 3D models of numerous implanted devices.
What are some of the advantages of having an in-house pathology team?
LF: The most obvious benefit is that our clients have access to everything they need at Veranex to take their devices from the early phases of surgical proof of concept to final reporting for regulatory agencies. Histopathology is key not only for the final assessment of devices to show how well they’re performing and how safe they are to use, but also for the initial stage of development. It provides valuable insights into the device itself. When the implanted animal shows signs of discomfort or health issues, histopathology evaluation can help uncover the reasons behind these concerns, such as potential device migration or heart valve leaks, offering a deeper understanding of the “why” behind the animal’s condition. Addressing these concerns early on is crucial as it allows for adjustments and improvements before entering the regulatory phase, where the design of the device is frozen. This proactive approach can prevent unfavorable reviews from regulatory bodies like the FDA, ensuring a smoother path to approval.
However, most of the time, the histopathological evaluation is performed at an external lab, without any connection with the team performing the procedure. This is not only inconvenient but also likely to leave gaps in the final assessment and reporting, which can compromise approvals.
We feel strongly about having our own in-house pathologic team because the histopathological exam happens in the same lab where the surgery is performed. This proximity is invaluable as it allows our team of pathologists to propose and develop personalized protocols tailored to meet our clients’ specific needs and required endpoints on a project-by-project basis. Organs are complex 3D structures, and being on-site enables us to expertly orient the cutting of samples, ensuring the production of 2D slices that provide a comprehensive understanding of the findings.
Our team of pathologists has access to the surgeons as well as the imaging specialists for the final report, which adds to the report’s depth as well as the ability for our client and regulatory authorities to easily understand and interpret the findings.
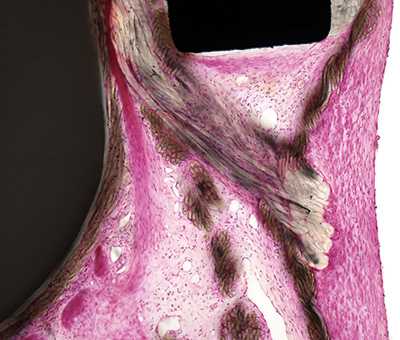
Ovine, Bioprosthetic mitral valve, PMMA resin section, H&E stain
We often hear how unique the Veranex pathology team is. Why is that, and what advantages does the team structure provide?
LF: Since we’re solely working with large animal models (pigs, sheep), we’ve intentionally hired vet specialists for all of our preclinical work. For example, vet surgeons, rather than surgeons trained on humans, perform the procedures, and our pathology department consists of four veterinary pathologists (me, Dr. Elise Guillaume, Dr. Corina Toma [fully board certified], and Dr. Gauthier Terrade [in residence]) and three dedicated histopathology technicians (Thibault Malnoe, Yasmine Lamari, and Maelle Hamzaoui). As veterinary pathologists, our comprehensive knowledge of the specific anatomy, physiology, tissue reaction, and spontaneous pathology of each animal species allows us to tailor study protocols to precisely meet the unique needs of every client’s project. We collaborate closely with our vet surgeons to conduct macroscopic assessments, perform tissue sampling, and comprehensively interpret the histopathological observations and integrate them with the clinical outcomes, imaging data, and macroscopic findings at the time of explantation. We can also collect and store samples, enabling us to conduct future analyses later in time, in accordance with our clients’ needs and plans. We’ve bolstered our team, allowing us to deliver pathology reports promptly. We now maintain a lead time of just three months from the explantation of the last animal to the initial report outline.
Because of this expertise and the opportunities for deeper analysis, we are confident that we can deliver the highest quality analysis and reporting for our clients.
You’ve mentioned collaboration between the vet pathologists and surgeons and its benefits a couple of times. Are there other ways that this collaboration contributes to the services the Veranex pathology department provides?
LF: Our end-to-end services include helping our clients optimize and tailor their protocols to measure the key endpoints and meet regulatory expectations. Our veterinary pathologists, veterinary surgeons, and study directors work closely together to ensure that the appropriate histopathology evaluations are planned as early as possible, often in the feasibility studies. We’re particularly qualified to provide this advice because of our familiarity with the advantages and limitations of each animal species as well as our extensive medical technology experience.
We’ve observed firsthand how delaying pathology involvement to regulatory studies can endanger product development. For example, if post-implantation degradation of a device coating is not detected in the feasibility study because of a lack of histopathological testing, there is no opportunity to address the issue in later trials, and there could be significant safety concerns from regulatory agencies.
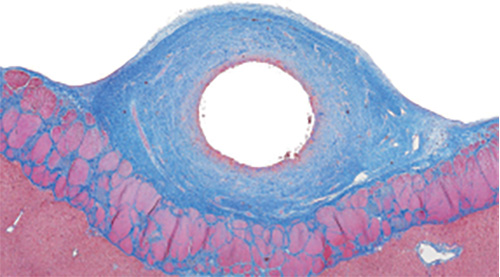
Ovine, Vena cava, Device passage, Paraffin section, Masson Trichrome stain
Our team has expertise not only in vet medicine but also with the techniques needed to support a wide range of studies. These include standard histological techniques, such as paraffin embedding, sectioning, and standard staining, as well as specialized applications such as frozen sectioning, MMA resin embedding of hard implants, EXAKT® cutting and grinding, special stains for qualitative assessment, and immunohistochemistry for specific cell or tissue component visualization. Many of these approaches allow us to more accurately evaluate the intimate relationship between the device and implanted tissue. We are also continuously learning and refining our processes for specific protocol requirements and endpoints.

Veranex’s Histopathology laboratory, Room #2
We have a long track record of successfully designing, conducting, and reporting studies to the FDA and regulatory bodies globally, and we believe our multidisciplinary approach is a major contributor to this success.
Reporting has also come up a few times in your previous responses. Can you explain the approach the preclinical pathology team uses for reporting?
LF: We want our clients’ devices to succeed probably as much as they do! As we know, success is dependent on more than just the evaluation and analysis. Our clients and, critically, regulatory agencies need to be able to easily evaluate whether the results demonstrate the level of safety and efficacy required to progress to the next development phase and ultimately to market.
That’s why we provide clear, richly illustrated, and didactic reports developed by the group of people most qualified to generate them — the surgeons and pathologists involved in the procedure and evaluation. The reports include narrative descriptions, qualitative and semi-quantitative assessments, individual data from large models, annotated microphotographs, and histomorphometry data, all supported by rich illustrations that make it easily understandable by our clients and the notifying bodies. The histopathology is complemented by the veterinary surgeon’s comprehensive understanding of postoperative recovery and the most likely cause (e.g., device or external event) for any postop findings, which adds a depth not present in reports from other providers.
We’re proud of our reports, and we often receive effusive feedback about how much our clients appreciate them.
Thank you for your time!

© Veranex Preclinical Services 2023. All rights are reserved. Prepared with: Prepared with: Laurence Fiette, Nicolas Borenstein, Cécile Jung, Renee Hoffman, and Jamie Sheard – Crossroads B2B Consulting.
Videos and previous newsletters on our services are available online here: www.veranex.com/preclinical-services/
