Veranex is proud to share the latest issue of our Preclinical Insights publication. In this issue we talk with Preclinical Paris Founders Nicolas Borenstein and Luc Behr on the changes they’ve experienced over the past 25 years, the legacy they’ve created, and trends they expect to see in the future.


Nicolas Borenstein and Luc Behr, Founders of Preclinical Paris
What have been the most significant changes in the industry over the last 25 years?
Nicolas and Luc: There have been many changes we have been so lucky to witness or even better to play a role in. First and foremost, I would mention the revolution of “structural heart” interventional cardiology, i.e., transcatheter aortic valve replacement (TAVR). It is truly revolutionary that, rather than a surgical approach, many people are treated for calcified aortic stenosis via a minimally invasive or fully transcatheter procedure.
It is bittersweet to think about those who would have benefitted from this incredibly ingenious innovation, including my (Nicolas’) late grandmother. Most clinicians never thought that treating aortic stenosis in this way would work. TAVR fueled many innovations to treat valvular disease via interventional means, namely for the mitral and tricuspid valves.
It is no chance, then, that the model we discovered as a very young laboratory helped to transform us into a professional service provider. The startup we worked with at the time brought together people from countries as diverse as the US, Israel, and France (Percutaneous Valve Technologies, acquired by Edwards Life Sciences).
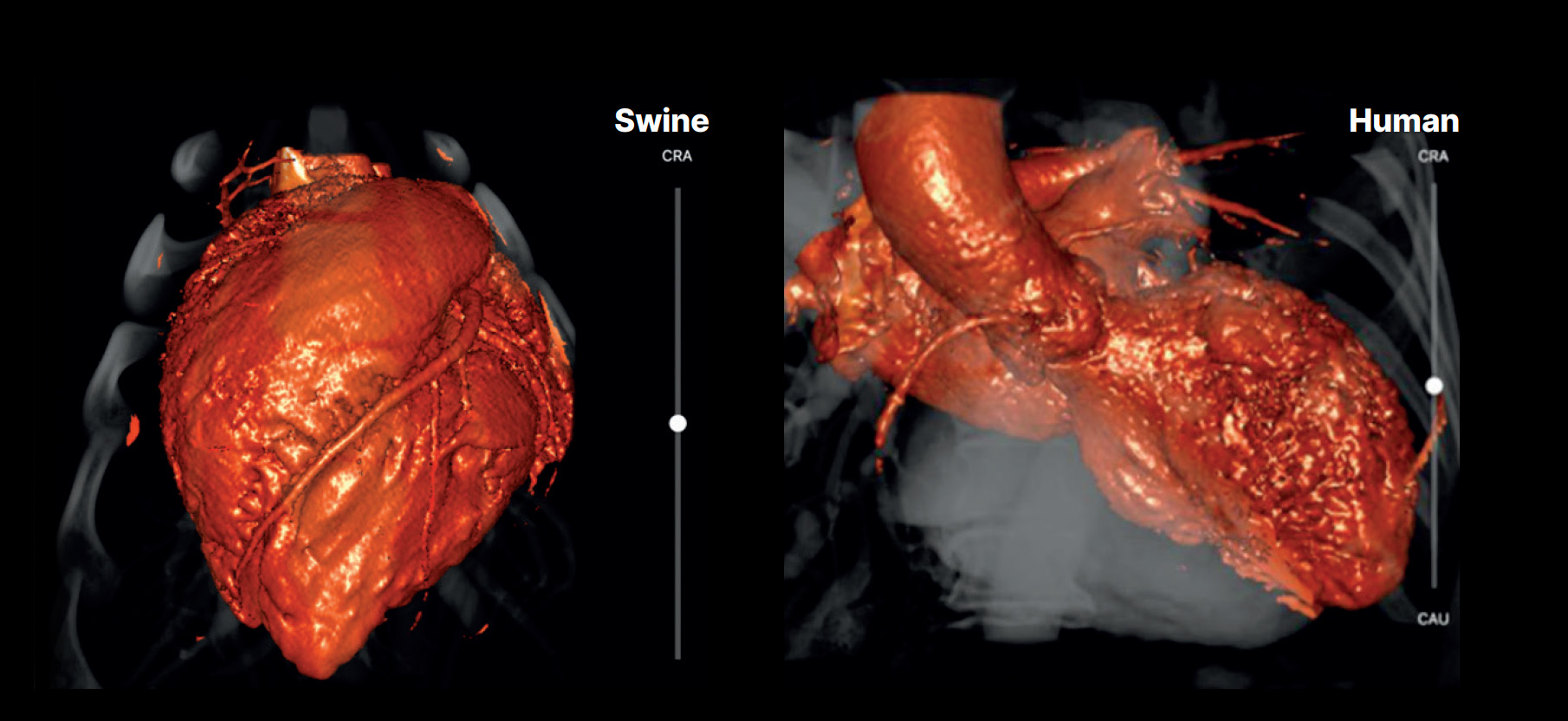
We’ve also seen imaging make a significant impact on the standard of care across treatments areas. In the field of cardiology, his includes echocardiography, with its vibrant, live 3D images of the inside of the heart to guide procedures, rotational angiography systems in integrated hybrid operating rooms (ORs), and CT scans, which provide insightful preoperative planning of complex procedures.
These improvements also fostered the dramatic development of interventional radiology in neurologic, vascular, and oncologic conditions.
Veranex Preclinical Paris’ Legacy in Numbers
Millions
OF PATIENTS TREATED TO DATE WITH MEDICAL DEVICES VALIDATED BY IMMR /VERANEX PRECLINICAL — PARIS
3000
PROCEDURES PER YEAR
3000
TAVI TO DATE
MORE THAN
5000
CARDIOVASCULAR AND THORACIC INTERVENTIONS
MORE THAN
3000
DEVICES FOR ABDOMINAL SURGERY IMPLANTED
MORE THAN
2000
NEUROVASCULAR AND VASCULAR PROCEDURES
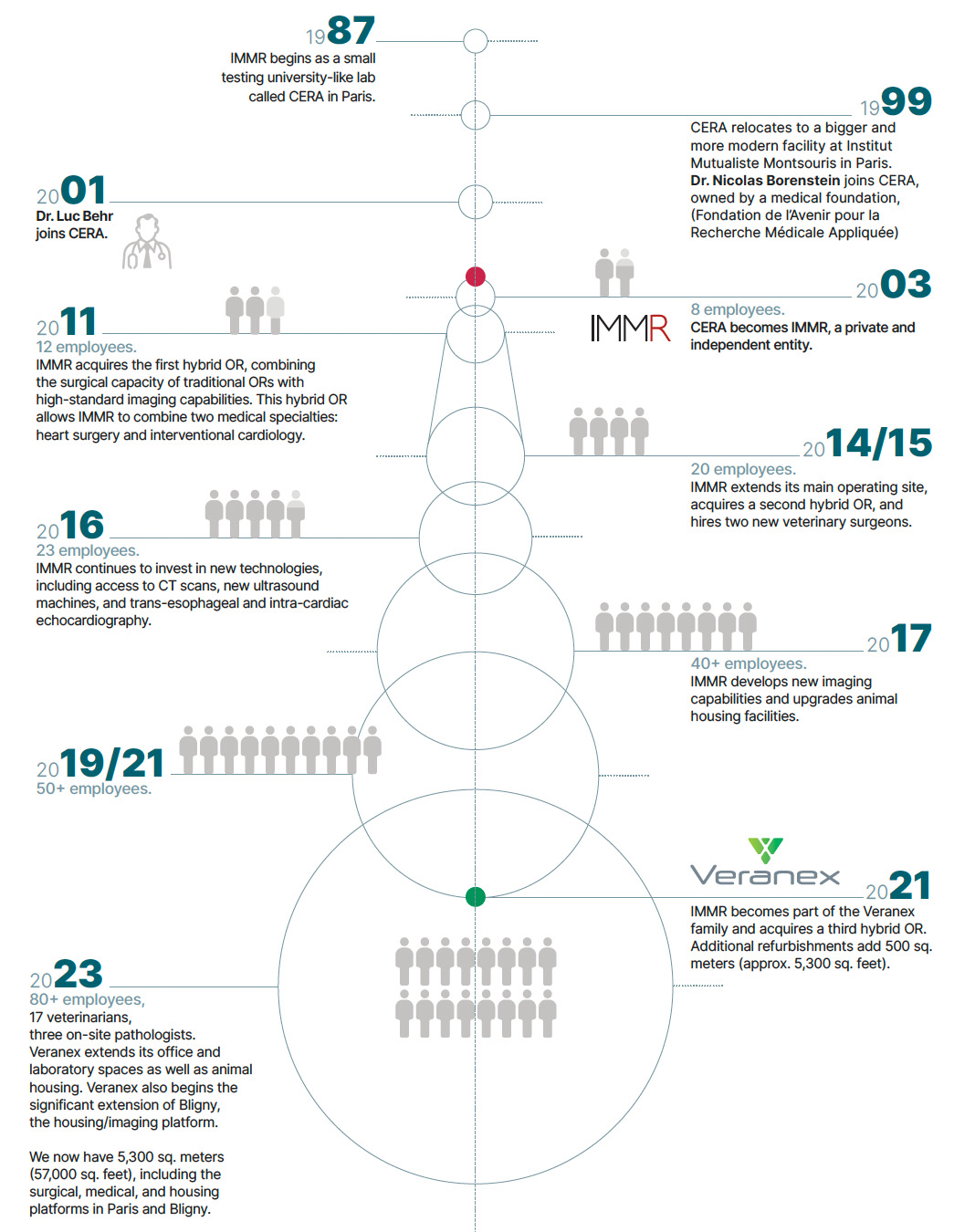
Timeline

I have been involved in testing and developing numerous medical devices with multiple companies during the last 15 years.
It has always been a fantastic experience to work with this extremely professional team of IMMR (now Veranex).
It is not only the superb experimental setup with state-of-the-art imaging but also the outstanding “before” and “after,” which is critical in chronic animal experiments.
There are not many facilities of this kind in Europe. I’m always looking forward to more projects at this unique facility with a fantastic team.
Are there any trends/changes you expect to see over the next few years/2024?
Nicolas and Luc: We expect to see more minimally invasive percutaneous and endovascular treatments, more image-guided procedures, more fusion between images and reality, and the miniaturization of devices. In the field of heart failure, assist devices will likely mature enough to become more widely accessible. The interconnectedness of the nervous system and other functions will also become a more significant focus, leading to a better understanding of the human body. Pioneers in the endovascular field will explore new materials to provide longer life expectancy and fewer side effects such as thrombogenicity and inflammation.
Pathology Image
Additionally, we are convinced there will be an exponential development of wearables, providing instantaneous information and contributing to better prevention of diseases. Artificial intelligence (AI) will also have a large part to play in the future, but there might be some time before this becomes a truly accepted medical reality.
What are the most memorable projects or devices?
Nicolas: When I joined this team 25 years ago, I saw an interventionalist successfully implant the first stented valve in a lamb. This device would become the Melody, eventually acquired by Medtronic. A year later, I was involved in the first implantation of the Sapien, the very first TAVR. Pr. Alain Cribier, MD—a remarkable physician, innovator, and celebrated interventional cardiologist was ecstatic. We all knew at that moment that we had seen history. I have many other fond memories of successes and failures from which we took precious learning.
The relationship and camaraderie we have with our clients in the OR is one of the true pleasures of our work. As I often say, we sweat together, rejoice, and cry together. As a co-founder and now a more seasoned veterinary surgeon, seeing my apprentices become masters is also a particularly fulfilling moment.

From the deep basement of the hospital we trekked arriving
from the Rouen, the US and Israel to meet and explore a novel concept.
We failed so many times. But with the partnership of Dr. Borenstein, the persistence of Dr. Cribier and Dr. Elchininoff, a new procedure arose; TAVR.
Congratulations to IMMR/Veranex on 25 years!
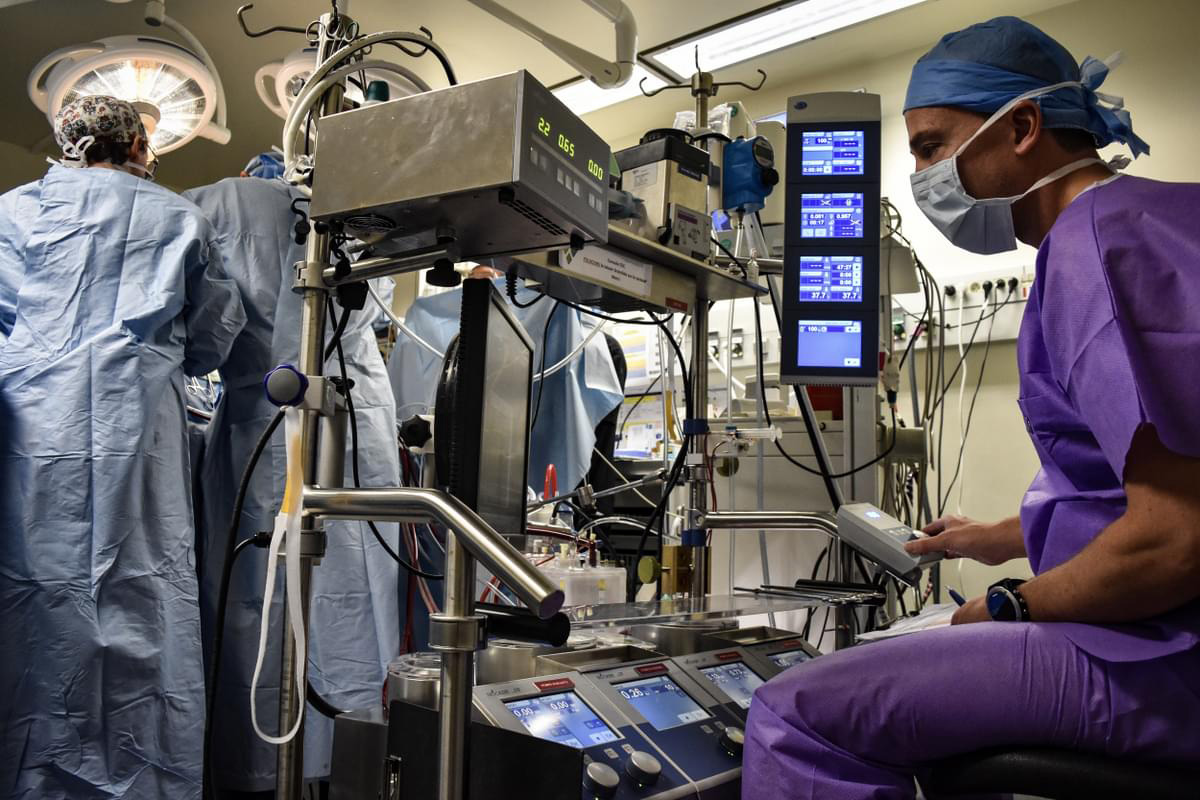

The 25th anniversary of IMMR/Veranex deserves to be warmly celebrated. I deeply enjoy being associated with the commemoration! What has been achieved at IMMR/Veranex over 25 years is absolutely remarkable!
The IMMR/Veranex team has offered hundreds of physicians and scientists from around the world the most outstanding presence, advice, and environment. IMMR/Veranex has supported many essential research programs leading to innovative medical technologies.
I was among the first to benefit from your facilities and expertise, evaluating TAVI on sheep models in the early 2000s. I will never forget the very high and incomparable quality of your support.
I associate IMMR/Veranex, Nicolas Borenstein, Luc Behr, and all their collaborators with the great success of TAVI.
Thank you all, and have a great celebration!
What values have defined this lab’s growth, success, and reputation?
Nicolas and Luc: It was evident from the beginning of the adventure that, as a team, we shared values that are now recognized as best practices by our clients and regulatory agencies worldwide. These values revolve around three axes: Ethics, Excellence, and Team Spirit. Ethics is ingrained in our DNA—we were pioneers in establishing an ethics committee for our experimental preclinical activity more than 25 years ago. Now, that is mandatory. We have built a team of veterinary medicine experts and technicians for animal welfare, surgery, interventional procedures, anesthesia, and imaging.
Scientific and professional ethics are also vital in our company. Our ethics allow us to deliver transparent and relevant scientific results to our clients and regulatory bodies within a highly skilled environment, servicing our clients’ projects rigorously and diligently. Excellence is our mantra; it is intrinsically linked to innovation. We permanently seek to improve our knowledge, know-how, equipment, and facilities to stay at the cutting edge of our field.
Finally, without our teams, our research laboratory would not be recognized as the leader it is today. Every day, we work hand in hand with our collaborators and clients, embarking on the adventure together. They often say that they feel at home when they work with us.
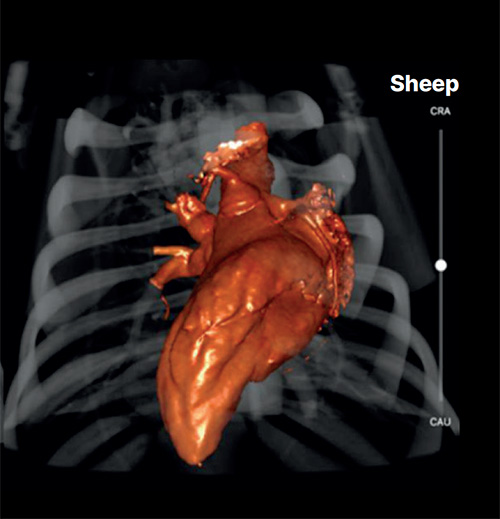

I was remarkably impressed with the quality of the facility and the expertise of the animal caretakers and veterinary surgeons at Veranex Preclinical Services in Paris.
Our experience was seamless: the animals were treated humanely and professionally, communication was excellent, the audiovisual services were second to none, and the animal surgery suites were as well equipped and maintained as my operating rooms in the United States.
We learned a great deal from our preclinical studies performed with IMMR/Veranex, leading to our further human product developments and our soon-to-be-initiated human clinical trials.
In the future, I will insist on returning to Paris and using IMMR/Veranex Preclinical Services.

Happy birthday, IMMR/Veranex!
What phenomenal growth in the past 25 years!
Having brought four early-stage MedTech projects through development and preclinical validation to you, I have always been impressed by the superb skillset of your team, its astute feedback and value added to product design and usability, and the flawless operations and execution of our studies.
Keep up the good work!
When IMMR joined Veranex, what vision did you have for the company?
Nicolas and Luc: We decided to join Veranex to address our clients’ needs for the entire medical device development life cycle. As a stand-alone laboratory, we experienced the limits of our expertise. With Veranex, we have partnered with world-class companies in regulatory, design and engineering, data management, clinical trials, and market access to bring efficient, integrated, cost-effective support to the entire medical device community.
What did you envision contributing to the medtech and biotech communities when you began? To science in general?
Nicolas: As a young surgical resident, I dreamt of bringing new surgical therapies to address the needs of patients. As it happens, 25 years later, we have hugely contributed to the evolution of surgery towards minimally invasive techniques such as catheters, robots, or video-enhancements. We can treat patients who were previously ineligible for surgery and faced poor outcomes. I am proud to say that along with the clinicians and engineers, we have saved lives because of the innovations we have brought from bench to bedside.

©Veranex Preclinical Services 2023. All rights are reserved. Prepared with: Nicolas Borenstein, Luc Behr, Renee Hoffmann, Amy Leiter, Brittany Kearns and Kristi Lee
Videos and previous newsletters on our services are available online here: www.veranex.com/preclinical-services/
