Veranex is proud to share the latest issue of our Preclinical Insights publication. Our state-of-the-art facilities in both Atlanta and Paris, innovative spirit, and passion for advancing medical technologies make us experts in preclinical vascular research, helping clients achieve success in their research and commercialization efforts.
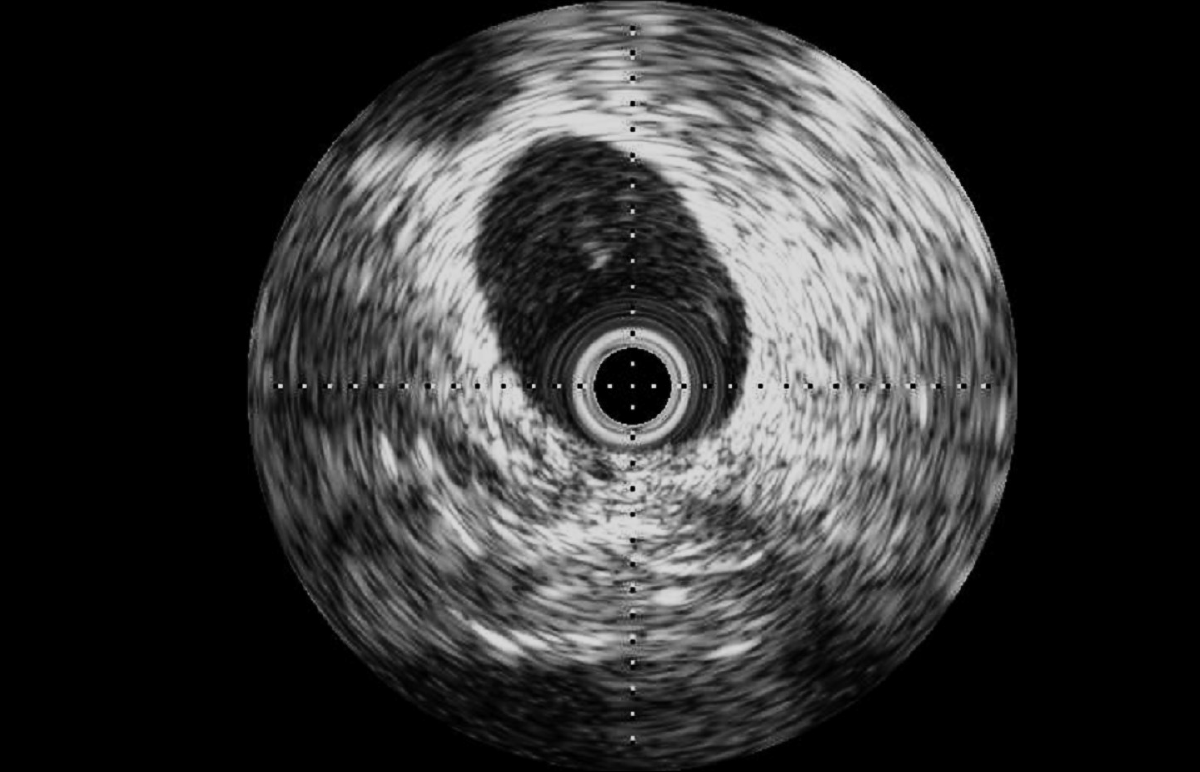
Intravascular ultrasound (IVUS): external iliac vein of a 100 kg-male ovine
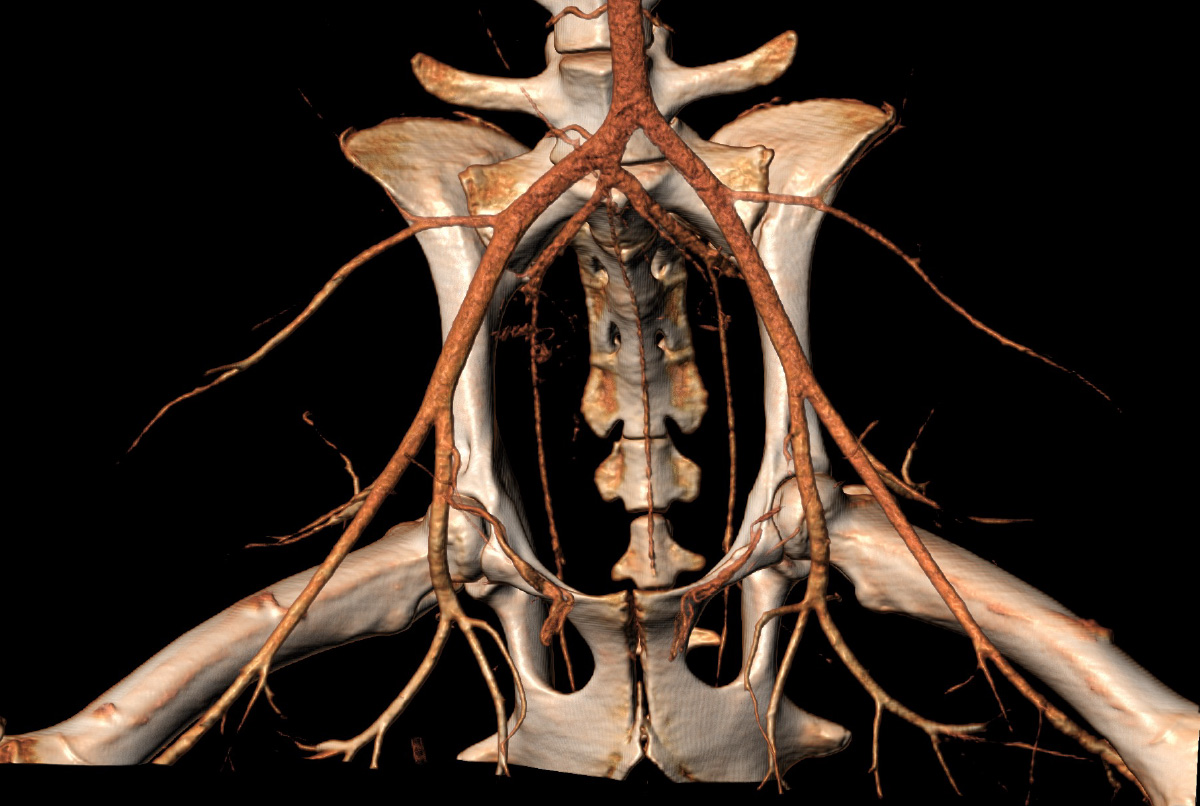
Computed tomography angiography scanner (volume rendering) of the terminal aorta in a 59 kg-female ovine
We’ve long pushed the boundaries of current knowledge to advance human medicine. When our team’s innovative spirit is combined with the right mixture of expertise and technology, we’re able to support our partners in developing vascular interventions for surgeons to provide the best possible care for their patients.
Our team, cutting-edge facilities, hospital-like technology platform, and available animal models, small and large, along with human cadaveric models embody the characteristics required to bridge the gap between the preclinical and human clinical environments. Using resources such as hybrid operating rooms and advanced imaging in a veterinary-led laboratory, our team has deep experience in vascular technologies, including coronary, peripheral, carotid, and neurovascular, to work at human scale in normal and pathologic models.
Preclinical large animal models can often more accurately represent the complex clinical and pathologic features of many human diseases, enabling translation to clinical practice.
Also, similarities in anatomy and physiology enable the use of advanced imaging and surgical procedures just like in humans.
The Veranex team has worked to develop extensive experience in creating models of deep vein thrombosis (DVT), chronic total occlusion (CTO), and below-the-knee and soft and hard clots that mimic clot compositions in human patients with complex vascular diseases.
Housing capabilities are essential for large animal model studies.
At Veranex, our preclinical facilities are capable of housing 1,100 large animals to support both acute and multi-year studies for multiple sponsors—without animals from different studies co-mingling.
Our facilities are accredited by AAALAC International and operate in compliance with the OECD Principles of Good Laboratory Practice (determined by the French national GLP compliance monitoring authority). Results from a single study can be used for submissions to multiple regulatory agencies because of our accreditations and attention to the requirements of all target markets from study start.
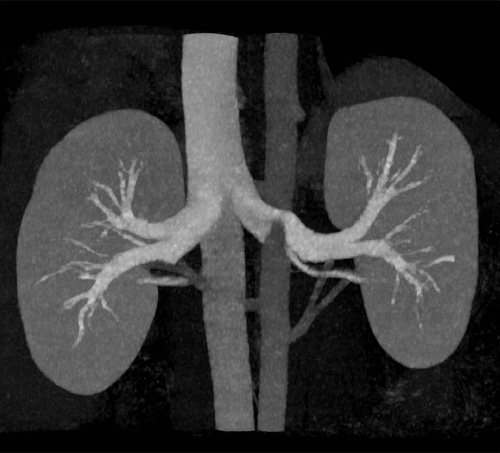
Computed tomography angiography scanner (MIP) of the renal vascularization in a 60 kg-female porcine
Disease models form the foundation of preclinical vascular evaluations.
A streamlined transition from preclinical to clinical evaluations relies on the ability to mimic the human condition as closely as possible while choosing the appropriate model is critical in developing vascular technologies. Small animal models can be useful at early stages of device development. Large animal models become necessary for size relevant clinical imaging orsurgical and interventional techniques. Furthermore conditions found in humans, which affect vessel size, elasticity, and compliance as well as blood flow,and remodeling, are challenging to design for small animals.
Our work in large animal models, such as sheep and pigs, has empowered important advancements in vascular medicine.
Advanced imaging ensures research delivers reliable results.
As part of our commitment to preclinical science and understanding vascular therapies, we’ve invested in the most advanced imaging technology possible. To inform and guide work we can be proud of, we use the following systems:
• Computed tomography (CT) for preoperative planning and postoperative imaging is used to assess anatomical structures, identify pathology, select the most appropriate model, and develop optimal procedural strategies.
• Fusion technology allows preoperative anatomic CT images to be overlaid with real-time fluoroscopy images, creating an augmented reality to facilitate guidewire and catheter navigation and stent placement.
• Non-invasive imaging modalities such as ultrasound and fluoroscopy provide real-time imaging, making them a good tool for guiding minimally invasive procedures.
• Enhanced visualization with cone-beam CT (CBCT) and/or fluoroscopy, complemented by advanced software available in our three state-of-the-art hybrid operating rooms, greatly enhances our ability to visualize detailed anatomical structures during angiographic procedures. It provides high-resolution, three-dimensional, cross-sectional images for optimal procedural strategies.
• Intravascular ultrasound (IVUS) may be a valuable tool to evaluate vessel morphology and size before implantation, guide device implantation, and assess long-term device position and safety.
• A complementary technique, optical coherence tomography (OCT) is an imaging modality that uses near-infrared light to provide high-definition images with higher spatial resolution and tissue penetration than IVUS, and our imaging specialists can help determine which technology is best for your needs.
• Non-destructive X-ray analysis (Faxitron MX-20 and Faxitron Hyperfocus Radiography Systems) enables ex vivo X-ray imaging of explanted stents and valves. Additionally, it facilitates analysis of images of biologic material and the integrity of metallic devices.
Our facility’s technology also allows us to better support our clients who might not have the capabilities to perform a procedure in-house. Because we have a high-end equipment platform available, our veterinary surgeon can perform the surgery and capture the information required for evaluation and submission. This is all crucial to Veranex’s mission to be a single-solution provider across the entire development lifecycle from concept of a medtech device innovative idea through commercialization.
Computed tomography angiography scanner (MIP) of the renal vascularization in a 60 kg-female porcine
“The guys at Veranex Preclinical Services always arrive at innovative solutions for the most demanding problems and are invaluable sparring partners for scientific discussions, enabling fast-paced progress for our projects. But apart from all this maybe the most wonderful thing is that going there always feels like coming home to friends.
Je vous souhaite un joyeux anniversaire !”
Accurate pathology evaluation is critical for timely completion of studies.
Identifying and classifying post-intervention microscopic vascular changes are key to understanding the acute and chronic effects of a surgical intervention in both vascular structures and the device. The composition of many medical devices is not amenable to paraffin embedding, so resin embedding plays an important role in the pathology evaluation. At Veranex, we have the specialized technical lab expertise and equipment to perform resin embedding, something we consider a key differentiator for our practice.
Again, imaging contributes to the assessment by providing spatial context. Non-destructive methods such as microCT, which we have available to us via key partners, reveal insights not available through the conventional pathological diagnostic process. We also use scanning electron microscopy to characterize developments such as calcification on the device.
If you want to learn more about how our pathology sets us apart, read the interview with Dr. Laurence Fiette, Veranex’s Head of Pathology, in our November newsletter.
Innovation relies on collaboration.
You can see our team’s collective passion for innovating
in healthcare in the facilities we’ve created around us. The right people, processes, and tools allow us to address the “unsolvable” problems and develop new solutions for people — and animals — in need. Our clients, partners, and peers are instrumental in helping us reach our goals, and their transformational ideas and tireless efforts continue to inspire us to help bring their concepts to reality.
We’ve been privileged to partner with companies large and small, including Becton Dickinson, Veryan, LimFlow, Aortyx, Robocath, Stentys, Vetex Medical (now SurModics), Neuravi (now Johnson & Johnson MedTech), and Boston Scientific, to name a few. Together, we’ve evaluated vascular technologies including coronary stents, abdominal aortic aneurysm (AAA) stent grafts, angioplasty balloons, thrombectomy devices, bioresorbable scaffolds, embolization devices, and more.
About Veranex
We have 140 staff members across our European and North American preclinical facilities including 21 full-time veterinarians, seven study directors, a dedicated team for training and education, board-certified veterinarian pathologists, quality assurance managers, medical writers, laboratory technicians, and a fully dedicated team to animal care
Our facilities are accredited by AAALAC International and operate in compliance with the OECD Principles of Good Laboratory Practice (determined by the French national GLP compliance monitoring authority).
Results from a single study can be used for submissions to multiple regulatory agencies because of our accreditations and attention to the requirements of all target markets from study start.
Our North American and European preclinical teams bring deep preclinical medical device expertise to our clients’ projects with state-of-the-art facilities, and the ability to complete complex surgical and interventional procedures safely, effectively, and ethically to generate the We look forward to continuing to redefine the standard of surgical care with our partners today and in the future.
Please contact us at: preclinicalinfo@veranex.com

©Veranex Preclinical Services 2024. All rights are reserved. Prepared with: Laurence Fiette, Nicolas Borenstein, Renee Hoffman, and Jamie Sheard – Crossroads B2B Consulting.
Videos and previous newsletters on our services are available online here: www.veranex.com/preclinical-services/
