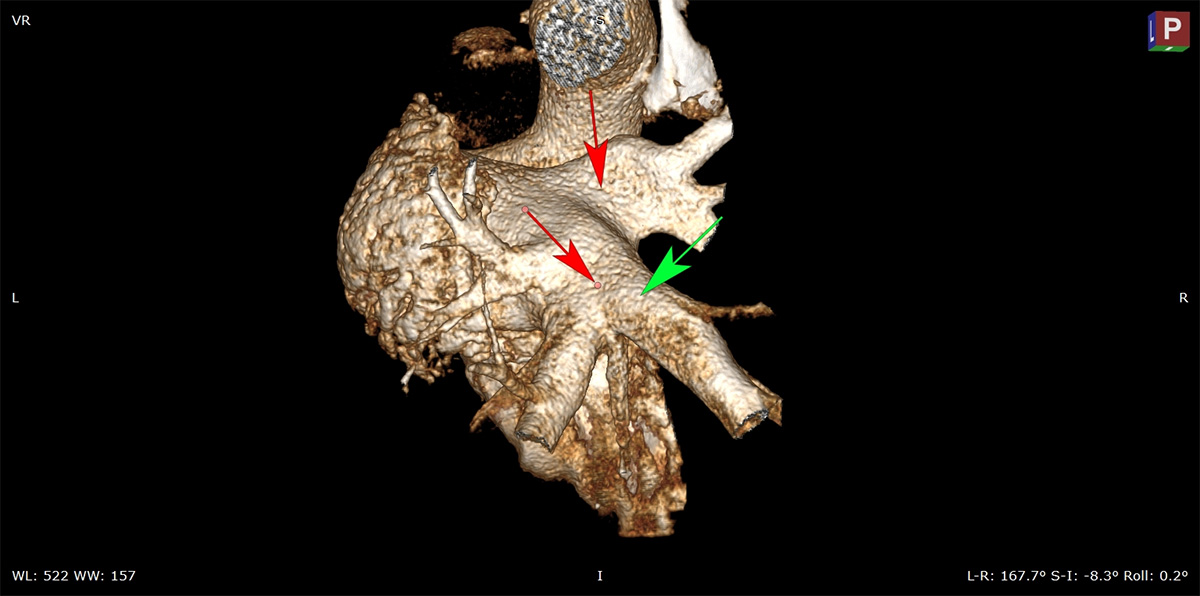
CT Image of Pulmonary Veins
Atrial fibrillation remains a leading cause of death and disability and the most common type of arrhythmia. While radiofrequency ablation and cryoablation are two established methods of treatment, in recent years more research has been dedicated to Pulsed Field Ablation (PFA) due to its faster procedure times and lower risk to adjacent structures making it one of the most transformational areas in electrophysiology to date. However, the technology is not straightforward and there are myriad potential complications that are not immediately obvious. Consequently, preclinical research plays a critical role in the safe and effective advancement of this technology. Studies must be carefully planned and rigorously conducted in appropriate models by an experienced team. At Veranex, our preclinical team is made up of leading experts in this area who can guide our clients through the design, conduct and interpretation of PFA procedures and act as a catalyst for success.
Below, preclinical study director and expert in electrophysiology, Irena Brants along with Drs. Robert Kieval, and James “Butch” Stanley discuss trends around PFA and how we work with clients to achieve their goals in developing this technology and procedures for use.

Irena Brants, DVM
Preclinical Study Director
Irena Brants is a veterinarian and a seasoned study director with over 17 years of experience in medical device preclinical research leading complex GLP studies in electrophysiology, interventional cardiology, neuro, structural heart, and other therapeutic areas. She brings expertise in preclinical study design, conduct, data interpretation and report writing for regulatory submissions.

James ‘Butch’ Stanley, DVM, MS, DACVP
VP, Research Pathology
Over his 23 years as medical device/biomaterial pathologist and pathology/scientific director in the preclinical CRO environment (most recently with HORUS Scientific, acquired by Veranex in April 2024), Dr. Stanley has worked closely with sponsors and investigators to guide them from the bench-top to the development and implementation of the protocols necessary for the successful histopathologic evaluation of their products for R&D, non-GLP, and GLP purposes. Dr. Stanley has deep experience with the development of the novel approaches required for the safety and efficacy assessment of emerging biomaterial and medical device technologies, regardless of anatomic location (e.g., cardiovascular, dermal, bone, neuro) or model (e.g., interventional, surgical, and disease).

Rob Kieval, VMD, PhD
EVP Business Development
Dr. Kieval has 20+ years of CEO and c-level leadership experience in health care and a track record of success in innovating, financing and commercializing sophisticated medical technology. His career spans the top-tier in medtech accelerators and start-ups, with positions including medical director at Medtronic and founder & CEO at CVRx. He has served on the board of directors of the Medical Device Manufacturers Association, as a director and board chairman at The Medical Alley Association of Minnesota and has testified before congress on behalf of the industry.
Current Trends in the Treatment of Atrial Fibrillation
Rob: What kinds of trends are you both seeing in the field of electrophysiology and treatment of atrial fibrillation?
Irena: I’m seeing a lot of companies transitioning away from radiofrequency ablation. In the last five years, there’s been a huge increase in development and research for PFA technology — especially at the preclinical phase, but also carrying into the clinical setting. Companies have just started getting FDA approval on PFA devices in the last three years or so and the outcomes have been impressive. In fact, Michael Mahoney, the CEO of Boston Scientific whose device was recently approved, said that PFA is the most transformational technology he’s seen.
Butch: I remember when I first learned of PFA for ablations, I thought it a brilliant use of that technology. As a medical device pathologist, there have been very few devices which I thought were truly different as a novel technology. From a tissue response perspective, PFA definitely offers some advantages over traditional ablative modalities (e.g., RF).
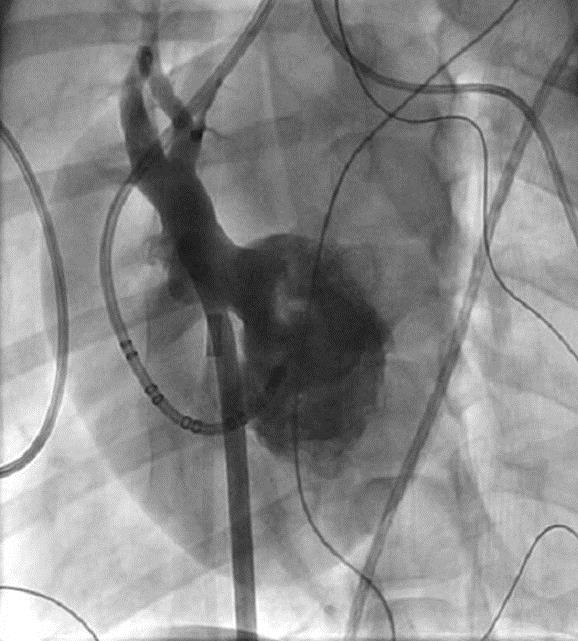
Fluoroscopy of Right Superior Pulmonary Vein
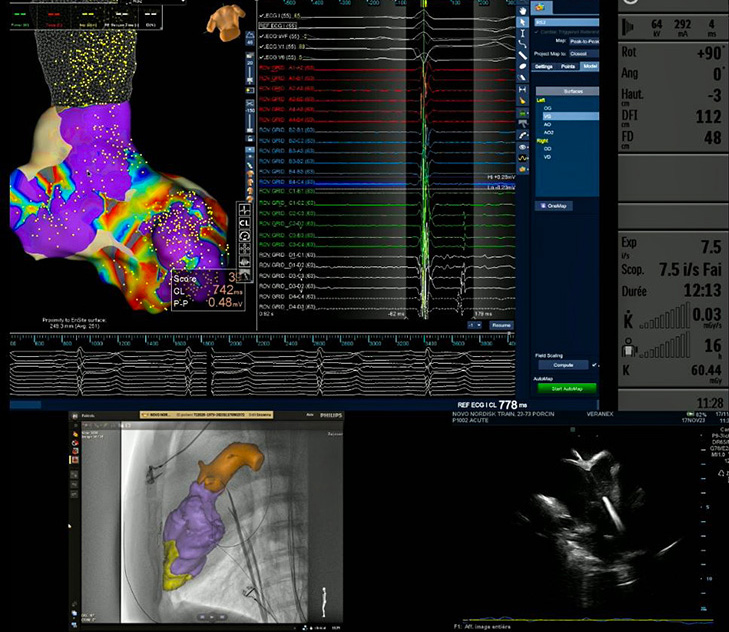
Imaging Set Up Required for Electrophysiology Procedure
Rob: With all the electrophysiology and PFA work being done here at our labs in Atlanta and Paris, are we primarily seeing early-stage feasibility studies or across the full R&D spectrum?
Irena: We see a variety of companies — some that are early on in their development looking for guidance with animal model selection, procedural workflow, or study endpoints, and then there are some that have done their non-GLP feasibility work elsewhere, have gained confidence in their device performance, know specific ablation parameters and treatment sites and are ready for a chronic safety and efficacy study in support of FDA submission. When clients come to us, it’s for the quick-turn, high-level, high-quality work that meets GLP standards and will stand up to the regulatory approval process.
Electrophysiology work that we do here involves participation of clinical electrophysiologists that may not always have preclinical experience and familiarity with animal models, anatomy. So, this is where our skilled team members come in to provide the support needed for successful outcomes.
Butch: I think PFA is ready for the market from a safety perspective, but there are still some refinements that need to happen for it to reach its full potential. Since its appearance in the preclinical space, I have performed numerous PFA ablation necropsies and generated GLP histopathology reports in support of PFA ablation studies submitted to the FDA.
The Cutting Edge: Working with PFA Clients in a Preclinical Setting
Rob: So, in our labs, not only are we able to complete these complex procedures fully and safely, given our depth of experience, but the success of our studies has also become more predictable as long as the technology performs as intended. Given the volume of procedures we’ve completed and the experience we’ve had across multiple different technologies we’ve helped to evaluate, we’ve already addressed many hurdles that our competitors and sometimes clients are still grappling with.
Irena: Right. So, when we work on these projects, sometimes our own interventionalists will perform the procedures, and other times outside surgeons and/or interventionalists contribute as test device evaluators. During the procedures, I make sure that everybody knows their role, understands the workflow and that protocol is being followed. It is great to witness special moments when physicians get to handle and use these novel technologies and devices, but important part of my role is to make sure they stay within the scope of work and don’t get carried away wanting to explore other features or modalities. With feasibility studies they have a bit more freedom, but for GLP studies, everybody involved must follow the protocol precisely.
Rob: What are some broader takeaways and critical success factors that you’ve gained in your time working on these kinds of procedures?
Irena: We’re learning from study to study to be better aware of and thus avoid potential risks, which allows us to accommodate sponsors and meet safety and efficacy endpoints outlined in the study protocol. We’ve been in the trenches and now we have ways to complete procedures without inducing arrhythmias, which is hugely important for studies that are fraught with cardiac and even non-cardiac complications that limit the ability to complete them successfully. Essentially, while others are starting from scratch, with the help of our veterinary experts here in Atlanta and in Paris, we’re on the cutting edge.
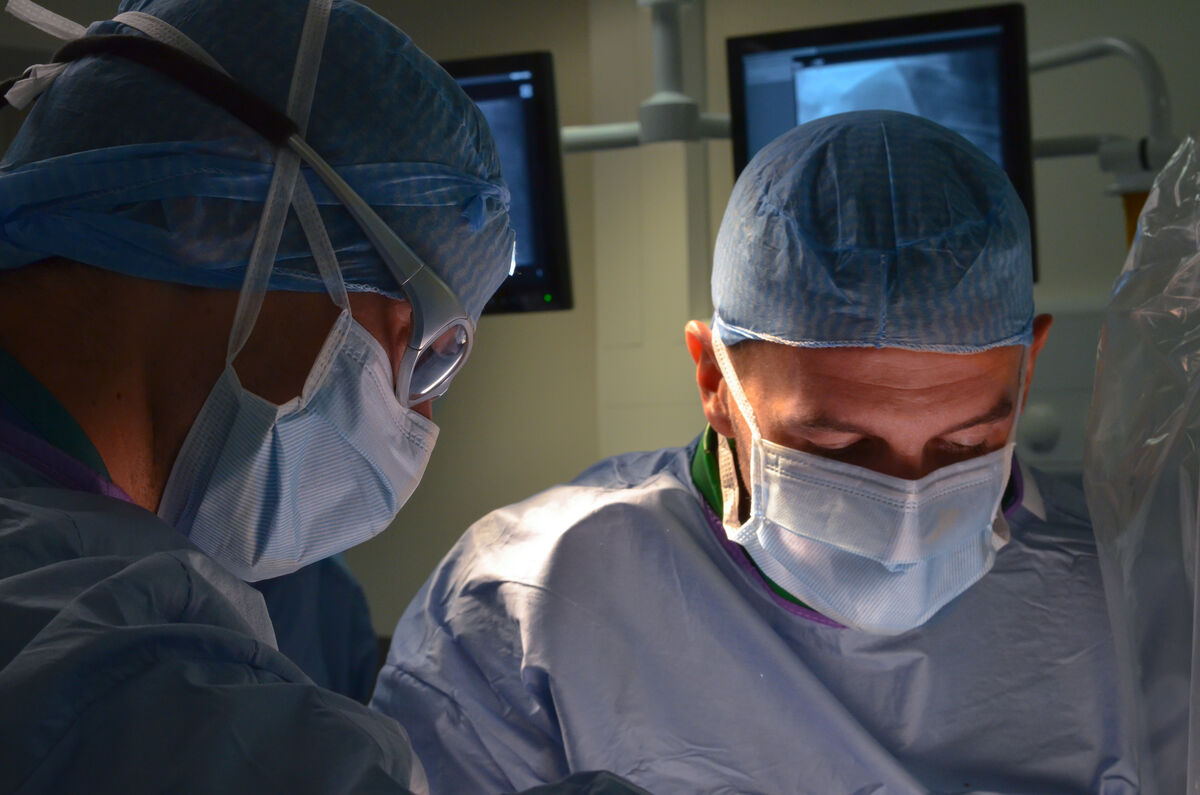
One other thing I’d add is the importance of pathology. We frequently collaborate with pathologists such as Butch who have deep experience in the field. They’re comfortable in our lab because our team knows how to prep, assist, prosect, examine tissues, take photos and not miss any critical structures or potential injuries.
Butch: Having been a visiting medical device pathologist routinely to Veranex’s Atlanta preclinical lab for many years, I can attest to the lab’s support for the needs of a device pathologist which allows us to focus on the target tissue. Also, as someone who spent most of his career within medical device CROs, I appreciate the excellent in-life care and procedural work that is performed by the Veranex team and the supportive and friendly culture they have developed. I am excited to once again be working in the CRO environment and couldn’t be happier that it is with Veranex.
Bright Future Ahead
Rob: Where do you see electrophysiology and PFA going in the future?
Butch: I think PFA is going to find functionality beyond cardiac ablation, for example, I could see its use in oncology for ablation of solid tumors.
Irena: I think it has a great future. Preclinical studies show that PFA treatment can be safe and effective for pulmonary vein isolation making it a promising option for patients with atrial fibrillation.
Rob: Thank you, Irena, and Butch for sharing your perspectives. Our sponsors who come to work with us in this burgeoning field are in good hands indeed!
