
Clinical utility describes the usefulness of a medical product (device, diagnostic/prognostic, or therapeutic) for physicians and/or patients, and key questions include the following:
- “Would physicians change their medical recommendations for the better (e.g., align with patient management guidelines) if they had access to the medical product?”
- “Would the medical product change the patient care plan and patient outcomes?”
For medical tests in the United States, the Centers for Medicare and Medicaid Services and other payers require clinical utility data for coverage and reimbursement decisions. Clinical utility is one of several product features that must be evaluated and shared to demonstrate the product’s value and lay the groundwork for product acceptance and adoption.
Value Framework for a Diagnostic Test
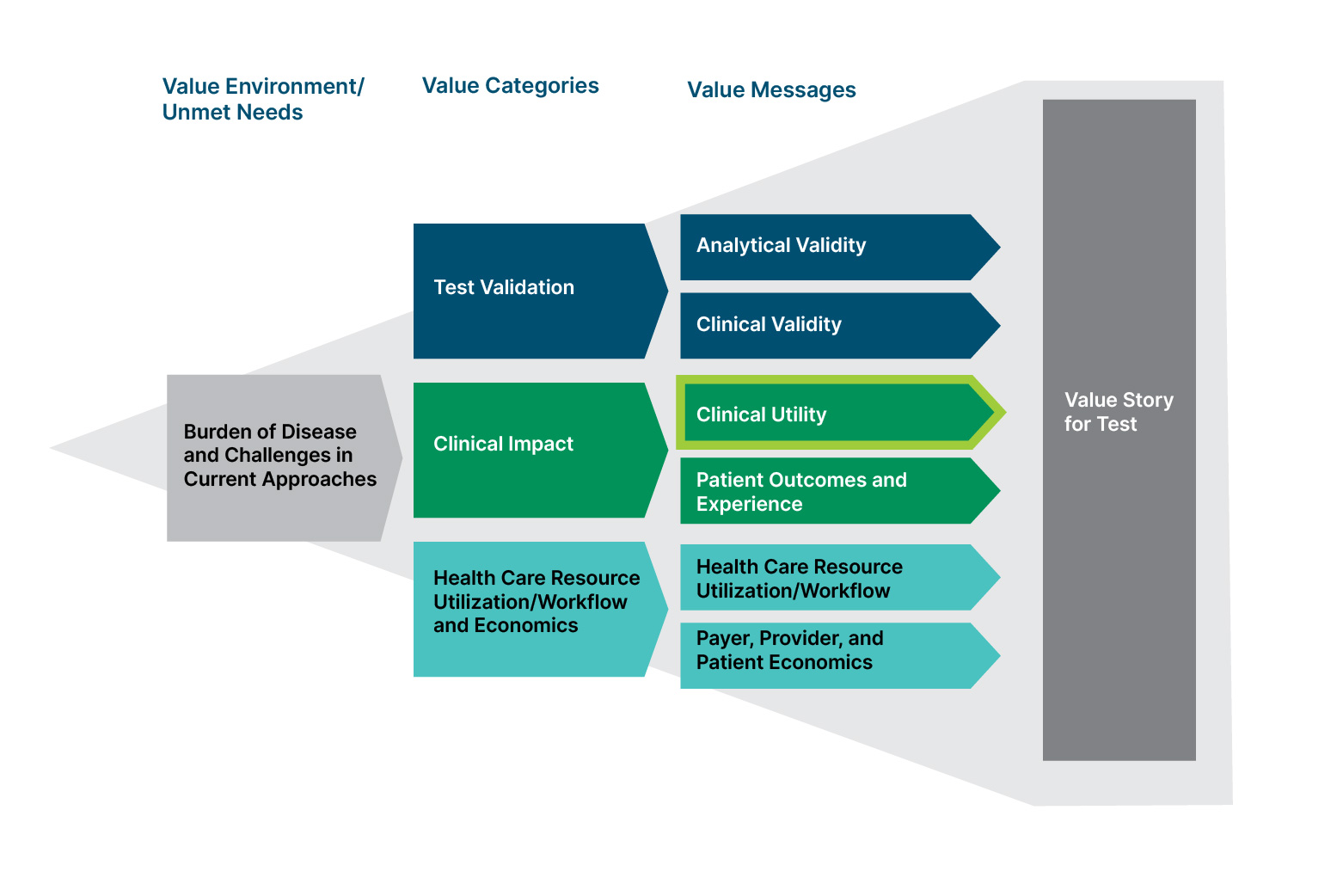
Clinical utility data developed by manufacturers can address the needs of payers, clinicians, and patients in several ways:
- Payers seek evidence supporting coverage and payment for new medical tests; clinical utility data can bolster the likelihood of positive coverage decisions by health insurers, and studies of clinical utility can demonstrate the superiority of a product in filling unmet needs in diagnosis/prognosis and treatment.
- Clinicians want to verify that new medical products improve patient care; information pertaining to patient outcomes and the ways physicians use and value products can be shared with the broader clinical community as part of education efforts.
- Patients are interested in medical care that effectively addresses their condition; clinical utility studies can demonstrate tests’ abilities to affect key clinical endpoints.
Clinical Utility Evidence Development
To assess clinical utility, manufacturers can use several methods, each of which has pros and cons.

Developing clinical utility data is a multi-year endeavor. Test manufacturers may employ more than one type of clinical utility study throughout the evidence development process. The case study below for a lung cancer screening test shows evidence development occurring over more than a decade.
Clinical Utility Evidence Development Case Study: Test X
The challenge: quantify the decision impact of Test X to support a value-based pricing strategy and encourage physician interest and payer coverage in the United States.
Clinical Utility Evidence Strategy
- Start clinical utility research early
- Employ multi-pronged approach with studies of different durations and time/cost investment
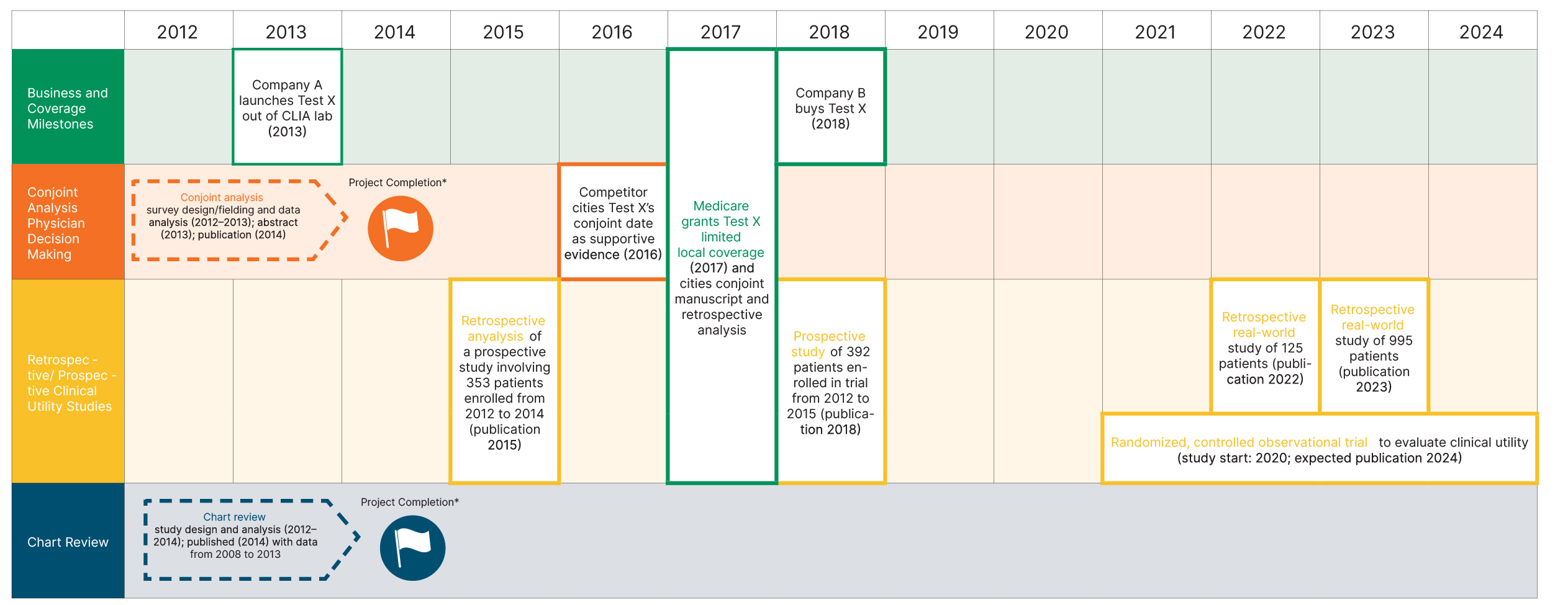
*Veranex was involved in the design and implementation of the chart review and the decision impact study (which employed conjoint analysis); Veranex also co-authored manuscripts describing these studies. Additional real-world evidence assessments, such as the large studies employed by the manufacturer of Test X, are now included in Veranex service offerings.
Results/impact: Statistically significant results demonstrated the clinical utility of the test in physician decision-making. When granting Test X limited local coverage, Medicare cited the conjoint analysis and retrospective analysis. Additional studies have been implemented to expand payer coverage and test use further.
Early-stage, shorter, and less costly studies (e.g., survey-based conjoint analysis studies, claims analyses, and chart reviews) can set the groundwork for payers’ positive coverage decisions and demonstrate to partners/investors the value of medical products. Later-stage, longer, and more expensive studies (e.g., prospective randomized controlled trials) can support widespread coverage.
Veranex has extensive experience helping companies develop and publish health economic evidence for medical devices, diagnostics/prognostics, and therapeutics.
For a list of recent Veranex publications and project experience, please contact Lauren Fusfeld, M.B.A. (lauren.fusfeld@veranex.com)
