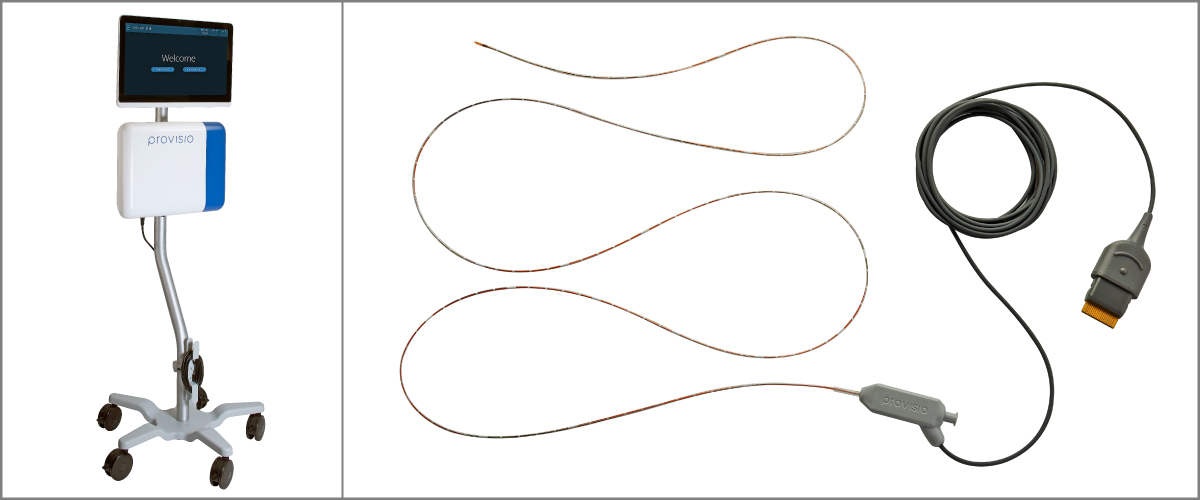
Provisio™ SLT IVUS™ System

Creating a novel medical device typically includes innovation around treatment, capabilities, cost reduction, or usability that results in improved patient outcomes. It also requires working with regulatory authorities to determine which qualifications must be met, and how to optimize the pathway to achievement. In this post, we speak with David Goodman (VP of Marketing at Provisio) and Robert Ashley (VP of Regulatory, Quality, and Clinical Strategy at Provisio) about developing a new kind of IVUS device, and the key steps the team took to obtain FDA clearance.
A Novel Idea
The regulatory team at Veranex partnered with Provisio to obtain FDA clearance for Provisio™ SLT IVUS™ System consisting of the SLT IVUS™ P1 System and the SLT IVUS™ Support Crossing Catheter This device uses A-mode ultrasound operation to evaluate the vessel lumen and provide dimensional measurements in real time. The information from the echoes is used to generate dimensions and representations of the peripheral vessel’s flow lumen. The cross-sectional parameters of diameter and area are displayed as numbers; additionally, the diameter measurements are displayed over time on a timeline display. As the operator moves the catheter through the vessel lumen, new cross-sectional measurements are obtained and displayed. Adjacent cross-sectional measurements can be compiled and displayed over a period of time to create an orthogonal graphic display of the vessel lumen. It also functions as a support crossing catheter and may be used for the infusion of saline or radiopaque contrast agents.
David explained the impetus for creating this novel device, “We recognized really quickly that there were three big impediments to traditional IVUS devices. One, the diagnostic configuration of traditional IVUS catheters can necessitate wire or other catheter exchanges which can be disruptive to the workflow and increase procedure times. Two, they can be expensive, and three, most people didn’t know how to interpret traditional grayscale IVUS images. With our device, which is integrated into a support crossing catheter, you’re instantly and accurately providing crucial measurements of the flow lumen of the vessel while navigating the vascular system, to facilitate making better clinical decisions.
Proving Accuracy
One of the greatest challenges the Provisio team had to overcome was proving the accuracy of the measurements that the device was putting out.
“There was skepticism during the medical review, about whether we could convincingly say that the numbers we were providing were an accurate representation of the vessel. Because, at the end of the day, physicians are used to looking at an image and measuring that image — like a baby’s head in an ultrasound. We’re just giving them the numbers — that’s a big leap of faith. What we had to do was work out a way that we could actually prove that the numbers were an accurate representation of the vessel,” explains Rob.
During the company’s FDA Pre-Submission meeting, FDA challenged them to scientifically demonstrate that the numbers the device was generating were valid. In bringing in Veranex, Provisio was able to leverage our cross-functional expertise in preclinical, usability, and regulatory to develop a validated solution that satisfied employees, investors, and most importantly, the FDA.
“Historically, Provisio was virtually structured on the regulatory side, with Veranex being the regulatory consultant,” said Rob.
Zane Liu, director of regulatory affairs at Veranex added, “The most challenging aspect was that this device differs from competitors. Usually, you get images in medical imaging. This is the first application of this type of ultrasound in this type of medicine. So, there was uncertainty from the FDA as this was super novel. We weren’t sure where the bar was in terms of meeting FDA regulations.”
While the road to regulatory success wasn’t straightforward, utilizing Veranex’s depth of expertise enabled Provisio to illuminate a path forward that remain aligned with their mission, business objectives, and commercial strategy.
“Veranex played an important role in convincing everybody that we needed to do usability studies. As would be expected for a start-up with limited resources, there was a lot of internal discussion regarding the necessity of these studies, but in the end, the technology was sufficiently different to existing technology that we all agreed the studies had to be done — it didn’t really slow us down at the time because we were still developing the system software and refining the catheter design while preparing the usability study protocols.
One of the things that the company should be very proud of is that we developed a pretty sophisticated cadaver model in order for us to be able to demonstrate the equivalence between the traditional IVUS technology and our technology. We were able to prove that we could accurately measure vessel dimensions in humans without doing human clinicals.
Partnering Up for Regulatory Success
When Veranex works with clients, we approach the project as a trusted partnership and our team becomes just as invested in the technology’s success as the client.
“Jennifer Gordon [director of preclincal quality assurance at Veranex] was extraordinarily helpful in putting together GLP studies and Naghmeh Nouri [executive director, quality engineering at Veranex] was absolutely invaluable in the usability studies. There was not a lot of recent experience doing usability studies in the company and the structure of everything that she put together along with the help that she gave us in understanding the extent and scope of what we had to do enabled us to do enough, but not so much that it became incredibly burdensome,” said Rob.
“Veranex is well-versed in helping small companies put their first products on the market and we’re unphased by novelties the industry hasn’t seen before. We have a broad range of expertise that extends beyond regulatory and compiling submissions. When the client needs it, we can tap into other resources such as preclinical, quality, and usability to ensure that the client has everything they need to succeed. Plus, we integrate really well with whatever teams they’ve already hired,” said Zane.
“Veranex is well-versed in helping small companies put their first products on the market and we’re unphased by novelties the industry hasn’t seen before. We have a broad range of expertise that extends beyond regulatory and compiling submissions. When the client needs it, we can tap into other resources such as preclinical, quality, and usability to ensure that the client has everything they need to succeed. Plus, we integrate really well with whatever teams they’ve already hired,” said Zane.
