Key Questions:
- Is the technology successful on bench tests of in vitro?
- Has a pilot study been performed to verify basic functionality and initial biological response before a GLP study for regulatory submissions?
- Are there design refinements that could be implemented?
Generally speaking, there are three types of preclinical studies and associated goals needed to advance the product lifecycle.
- Proof of Concept Whether you’re bringing a novel device to market or improving on existing technology, you should complete bench or in-vitro testing, then a preclinical proof-of-concept study. This will identify the optimal animal model, demonstrate trouble-free use, and confirm basic functionality.
- Feasibility After proving the concept in vivo, the next step before engaging in a GLP study is a small-scale non-GLP pilot study intended to further validate the proof of concept. This shows the benefits of latest refinements, reveals potential risks, and establishes replicable workflows and solid means of data collection.
- Safety & Efficacy Conclude your preclinical study design and cycle by proving the device’s safety and efficacy in a clinically relevant model of human disease along with GLP reports required by regulatory bodies.
Understanding the Study Process
Our sponsors journeys begin with our business development team. This team is designed to help you develop your preclinical program, including the optimal model. Selecting the right, clinically relevant in-vivo model is an essential component to the study’s efficiency and quality.
- If a large animal model is needed, are there any species-specific sizing, anatomical, or physiological variables?
- Is one or the other more significantly or more clinically relevant?
- What’s required for regulatory FDA review?
- What are the required endpoints, sample size, and target anatomy?
There are the questions that the team will answer based on many years of preclinical experience. For maximum efficiency, multiple separate studies may have to be initiated to provide clear-cut data, allowing you to stay on top of submission deadlines. Quotes, confidentiality agreements, and contracts defining the scope of work and protecting IP are also a result of this stage.
The scope or statement of work we expect from our sponsor usually includes:
Species and quantity (if known)
Timepoints
Deliverables (type of services, need for final reports, need for histology, etc.)
Any speciality supplies
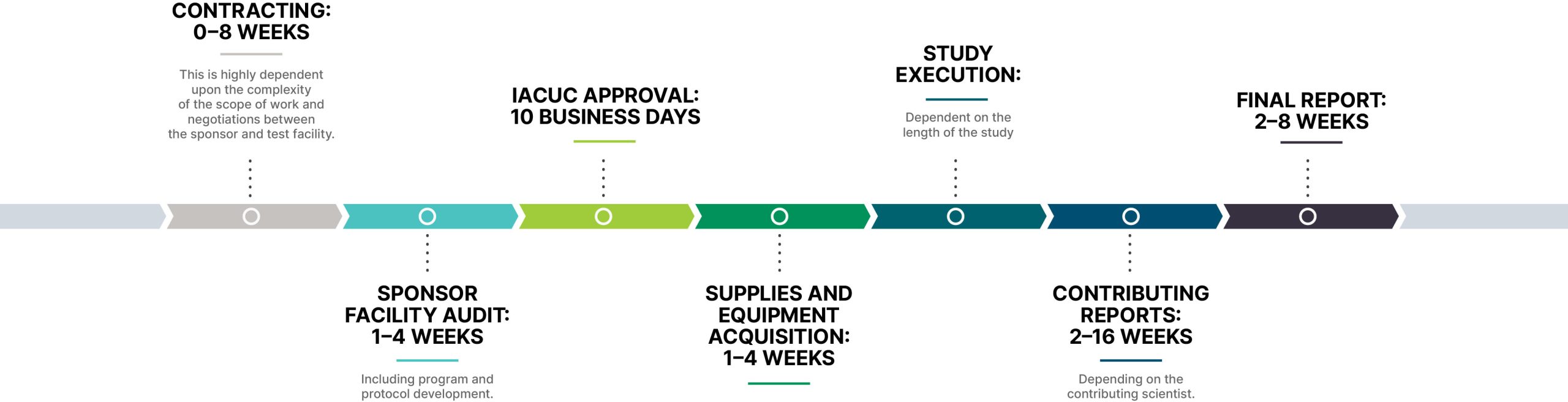
Setting Realistic Expectations in Data Acquisition
We have executed GLP studies to completion in as few as eight days. To be clear, that is the exception, not the rule. We have also executed years-long translational studies in the interest of determining very late responses to new devices versus the current standard of care.
Medical product developers should anticipate the following ranges, even in highly efficient preclinical studies, depending on predicate products, truly novel solutions, and the type of regulatory approval required.