Establishing a Strong Assay Foundation
Before we can conceptualize IVD product configurations, we must establish assay inputs which include, but are not limited to:
- Identify the need for the test, e.g. diagnose breast cancer or sepsis, identify bacteria, monitor glucose levels, etc.
- Identify the target biomarker or indicator, i.e. is there a biomarker or markers that are correlated to a condition or disease?
- Identify where the target can be found, e.g. urine, blood, saliva, etc.
- Determine the availability or frequency of appearance of the target; when are levels the highest?
- Determine if and how the target can be detected
- Determine sampling frequency, e.g. single sample or continuous monitoring
- Determine assay method stability
- Statistical information to confirm assay reproducibility
A well-developed and robust IVD assay will help to decrease the overall timeline and cost of a product development project. The first step, which is primarily driven by the demands of the market, is to determine which condition or disease monitoring is needed. After careful selection of a target and development of the assay foundation, you may move on to product design for concept generation, prototyping, and feasibility testing.
The Assay Development Pipeline
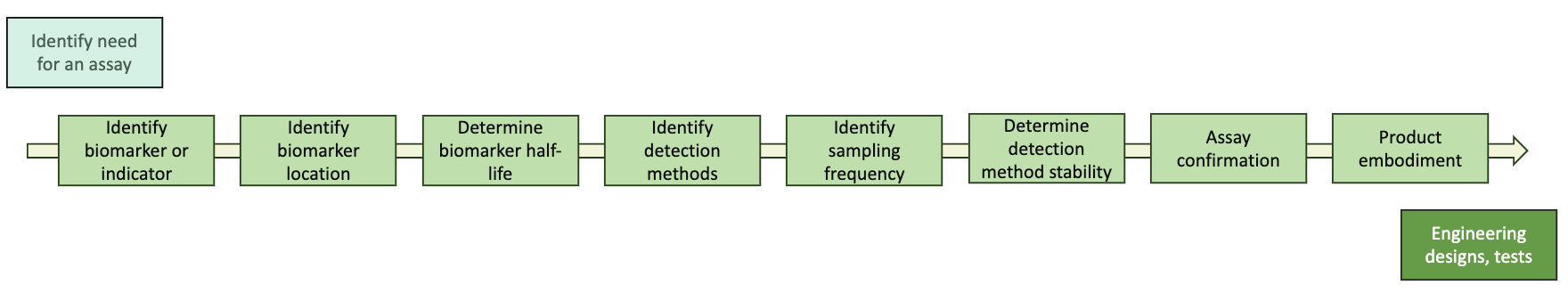
Identify the Target
- Determine which target biomarker or indicator is specific or directly correlated to the condition or disease of interest
- Rationale: Selecting the appropriate target reduces ambiguity when using an assay to diagnose a disease or monitor a condition
- There may be one or several associated biomarkers (e.g. RNA, DNA, antibody, small molecules, antigen, etc.)
- Selecting specific biomarker(s) or indicator(s) is critical and impacts assay sensitivity and specificity
Examples
- Human chorionic gonadotropin as an indicator for pregnancy
- Spike protein as an indicator for the presence of SARS-CoV-2
Identify Target Location
- Determine the biodistribution of the target biomarker or indicator
- Target may be found in multiple tissues
- Rationale: Selecting the appropriate sample type ensures capture of high concentrations of the target biomarker
- Targeting high concentrations increase signal-to-noise and can improve sensitivity
- Non-invasive sampling is typically favored and improves use adherence
Examples
- Sampling human chorionic gonadotropin from blood or urine
- Sampling SARS-CoV-2 from blood or a nasal swab
Determine Target Availability
- Determine the availability of circulating target biomarker or indicator
- Rationale: Some targets can remain available or persist in circulation for hours or days, or continuously at different concentrations
- Understanding what the limitations are and when to sample is critical to improving assay sensitivity
Examples
- Glucose is consistently present in the bloodstream, albeit in different concentrations
- Genes from SARS-CoV-2 are not consistently available and differ in concentrations
- Prostate specific antigen levels may increase as prostate cancer progresses

Identify Detection Methods
- Determine methods to detect or quantify the target biomarker or indicator
- Rationale: Selecting the appropriate detection method ensures that optimal sensitivity can be achieved
- Selecting the detection method is also influenced by commercialization goals
- Reagents that dilute or stabilize the sample of interest for a specific detection method require careful selection
Examples
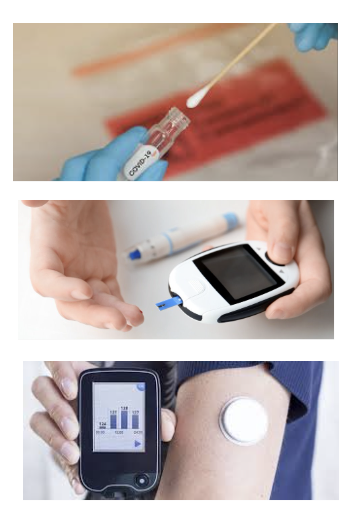
- Human chorionic gonadotropin can be detected using a single-use disposable lateral flow assay
- Nucleocapsid protein from SARS-CoV-2 can be detected using a single-use disposable lateral flow assay
- Genes for SARS-CoV-2 can be detected using polymerase chain reaction (PCR)
Identify Sampling Frequency
- Determine the frequency at which to sample the target biomarker or indicator
- Rationale: Understanding when to sample is necessary to ensure that the intended detection timeframe of target is captured
- Sampling frequency often correlates with target availability
- Sampling duration will also determine if a power source is required
Examples
- Proteins from SARS-CoV-2 can be sampled as needed; no power source required for a single-use lateral flow assay
- Glucose monitoring can occur continuously via a battery-powered wearable device

Determine Assay Method Stability
- Determine what aspect of the assay could impact product stability
- Rationale: Understanding method stability informs how long an IVD can be used or stored, and therefore, how the IVD is designed
Examples
- Assay reagents may be require automated mixing, which impacts product design
- Assay reagents may require cold storage, which impacts shelf-life and shipping
- Assay requires temperature-dependent incubation, which may require a small battery
Assay Confirmation
- Confirm that the assay is accurate and precise
- Rationale: A robust and reproducible assay will help to reduce the product development timeline and cost
- Statistical information is important, e.g. coefficient of variation <20%, 95% confidence interval, etc.
- Statistical information is also used to assess sensitivity and specificity
Examples
- Assay was able to detect cancer marker with a sensitivity of 90.6% (95% CI 81.9–99.2)
- ELISA inter-assay precision has a coefficient of variation lower than 10%
Product Embodiment Vision
- Design preliminary concepts for the IVD product
- Rationale: A vision of the product will guide the engineering team in their concept generation work
- Early concepts can be ideas, sketches or a prototype
- Design can feature enhancements, e.g. temperature sensor, sound
Examples
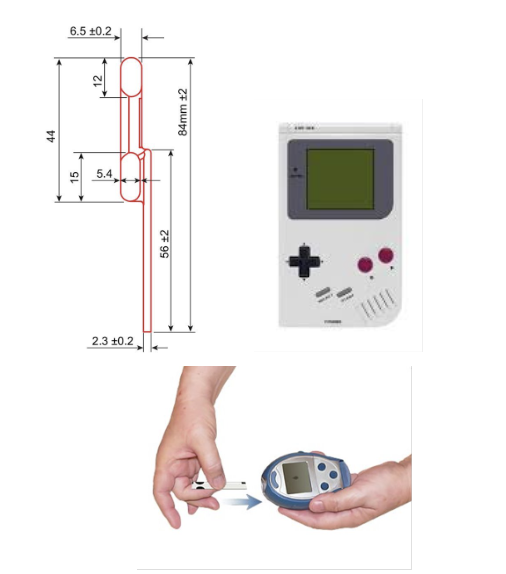
- Prototype of a microfluidic consumable
- Sketch of a high-throughput automated device to isolate nucleic acid
- Product embodiment wish list, e.g., “Size of a mobile phone” or “Water-resistant”