In the vastly dynamic clinical research industry, staying abreast of industry changes, fine-tuning the solution based on the trial complexity, and handling challenging timelines, all while ensuring the documents meet regulatory and health authority standards, represent a mammoth task. Veranex is committed to providing sponsors with documents that have been through a rigorous quality control (QC) review process by our in-house document review team.
A detailed, structured QC review is crucial to the development of accurate, consistent, and reliable medical writing documents for regulatory submissions. Our Clinical Data Services (CDS) document review team has the technical expertise in, proficiency at, and a thorough understanding of data quality review. The team works closely with the medical writers to understand their requirements and provide documents reviewed for data quality that meet the highest standards in the industry.
The Veranex document review team has extensive experience at performing QC on an array of regulatory submission documents for complex trial designs and analytical structures. These documents include protocols and protocol amendments, clinical study reports (CSRs), development safety update reports (DSURs), investigator’s brochures (IBs), clinical summaries (Module 2.7.1 through Module 2.7.4), clinical overviews (Module 2.5), integrated summaries of safety (ISS), integrated summaries of effectiveness (ISE), and safety narratives.
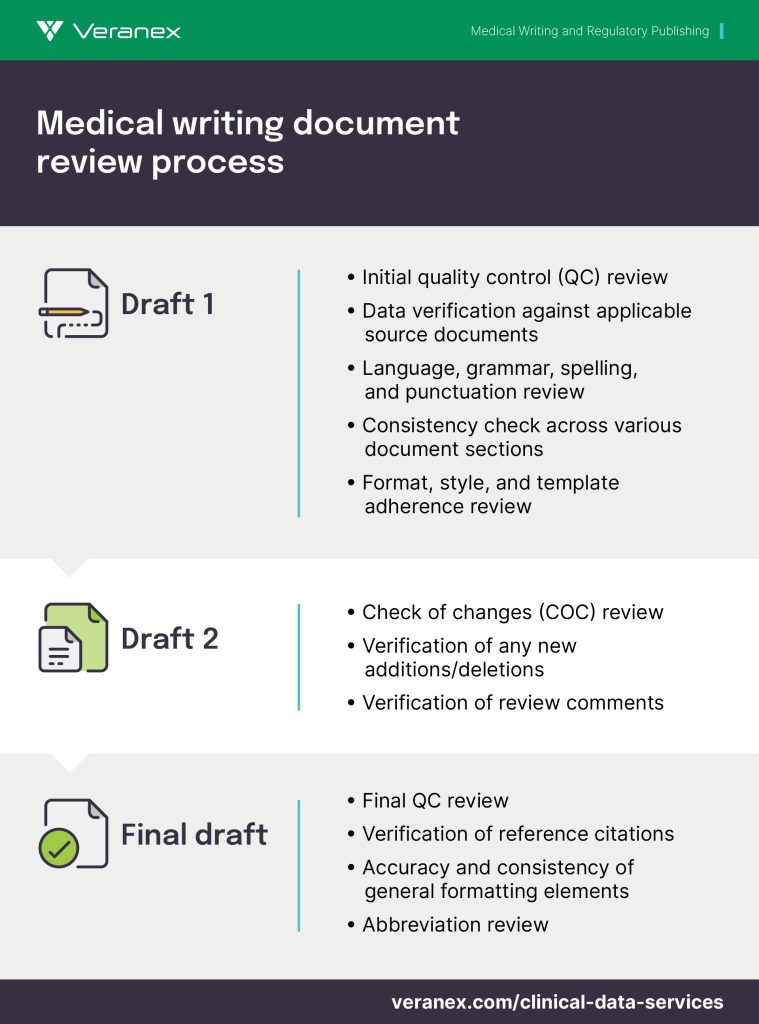
The QC review process at Veranex is scheduled at each stage of document development or as per sponsor requirements and consists of:
- 100% data validation against the source
- Consistency within the document and with the source
- Checks for incorporation of sponsor requirements and suggestions in the document
- Review for grammar, punctuation, and abbreviations
- Comparison against the AMA Manual of Style or the sponsor’s style guide and preferences
- All other checks per the Veranex/sponsor QC checklist
Veranex’s in-house document review team ensures that consistency and accuracy are achieved within a document with the aim of successful approval by regulatory agencies.
Contact us to discuss how we can support your Medical Writing needs.
