Extending Patient Lifespan with LifeSTEM
What is Glioblastoma?
Gliomas are brain tumors that come in multiple forms ranging from low-grade, benign tumors to high-grade, malignant tumors including glioblastomas. Glioblastoma treatment involves surgical resection, chemotherapy, and radiation. However, these tumors are typically incompletely resected, so there is a 90% recurrence rate [for a course of treatment, care and procedure costing approximately $200,000]. While implantable devices currently exist for the delivery of drug-based therapies, there is no device designed for the delivery of cell therapy to the brain to treat gliomas. This presents a challenge as the initial administration of cell therapies is done in the operating room upon surgical resection, and another invasive surgery is required if another round of cell therapy treatment is to be administered.

To address the challenges associated with treating Glioblastoma, Team AdvanCED developed LifeSTEM — a revolutionary neurological implantable device designed for the local delivery of stem cells to treat patients with brain tumors.
LifeSTEM utilizes a multi-chamber reservoir for targeted, local delivery of the therapy, a guiding sleeve, and catheters that are customizable in length to account for variability in patient anatomy and tumor location, as well as perforated catheter tips to allow for radial diffusion of the therapy and minimized odds of potential reflux. This device has the potential to reduce the risk of additional surgical procedures and extend patient lifespan.
The team’s focus was on the delivery of mesenchymal stem cells revealed the tip of the iceberg in regards to the potential in this space. Expansion could include delivery of CAR T-cells, extracellular vesicles, nanoparticles, microRNA, drug therapies, as well as other neurological and non-neurological diseases.
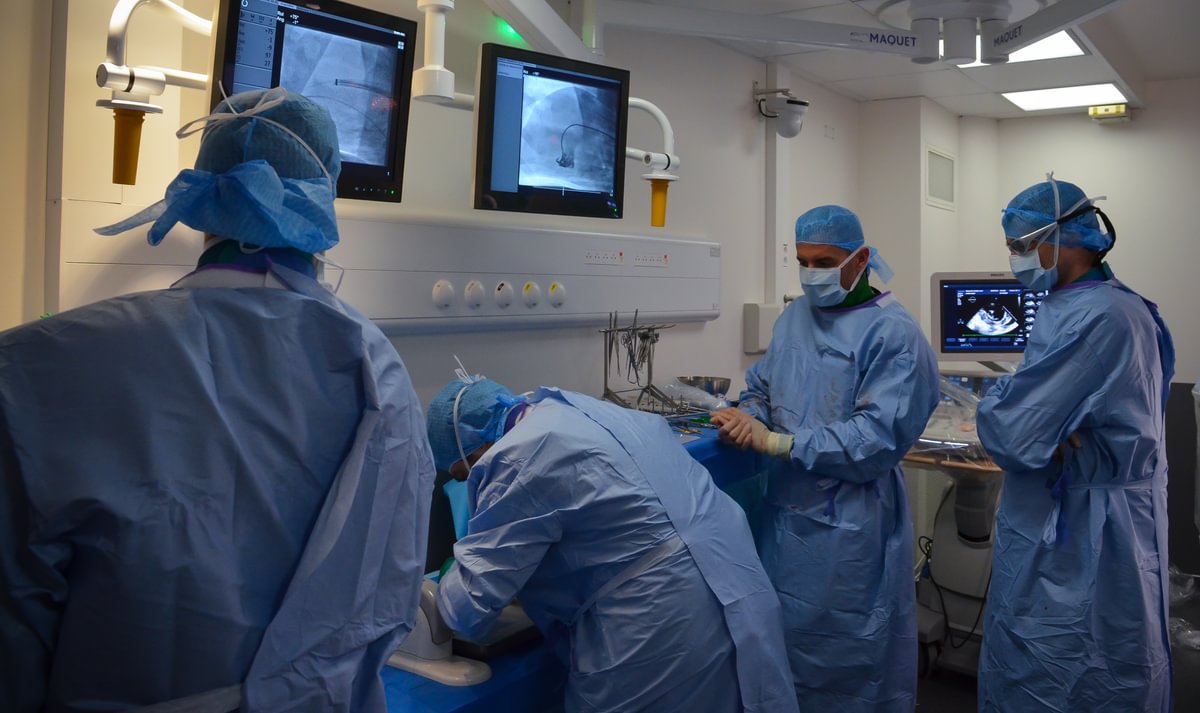
This project originated from a problem statement provided by Mayo Clinic. While working with Mayo, AdvanCED received crucial access to the surgical team and research fellows who provided them with information on the properties of stem cells, including fixed-state cadaveric testing.
The Solution
Our preclinical staff helped AdvanCED measure contrast flow rates and diffusion patterns or signatures for their device in a cadaveric porcine brain with properties much more like a neurosurgeon and team would experience when treating people fighting cancerous gliomas.
“In addition to supplying the organ, with its properties much more akin to what a surgical team would encounter, the [Veranex] team helped us gain high-value imaging thanks to the onsite, in-OR c-arms and operation and observation of the infusion pump to ensure the desired flow rates were achieved for the entirety of the test duration,” said AdvanCED team member Isabella Varea. “This help, along with the preparation and set up, gave us enough of an idea of how the device actually works in a more realistic clinical scenario, how it can be improved, and how the team at Mayo can effectively carry what we’ve learned forward.”

The team submitted submitted its results to Mayo as proof of concept. They believe, based on the positive feedback from doctors they worked with and the Mayo regulatory board, that Mayo will forge ahead with additional grant applications and a patent application relying heavily on AdvanCED’s work — work that will be intensive given the likelihood of an FDA Class III regulatory pathway for the technology.
