AmplifiDx
Next-Generation Point of Care Platform
From Concept to Functioning Prototype in Under 90 Days
Services
Industry
The Challenge
Develop a molecular point-of-care instrument using isothermal detection and lamp amplification with a first proof-of-concept completed in under three months.
The Solution
AmplifiDX partnered with Veranex to leverage our broad range of product design and development capabilities, along with our proprietary pre-built technology to achieve the three-month milestone on time and on budget.
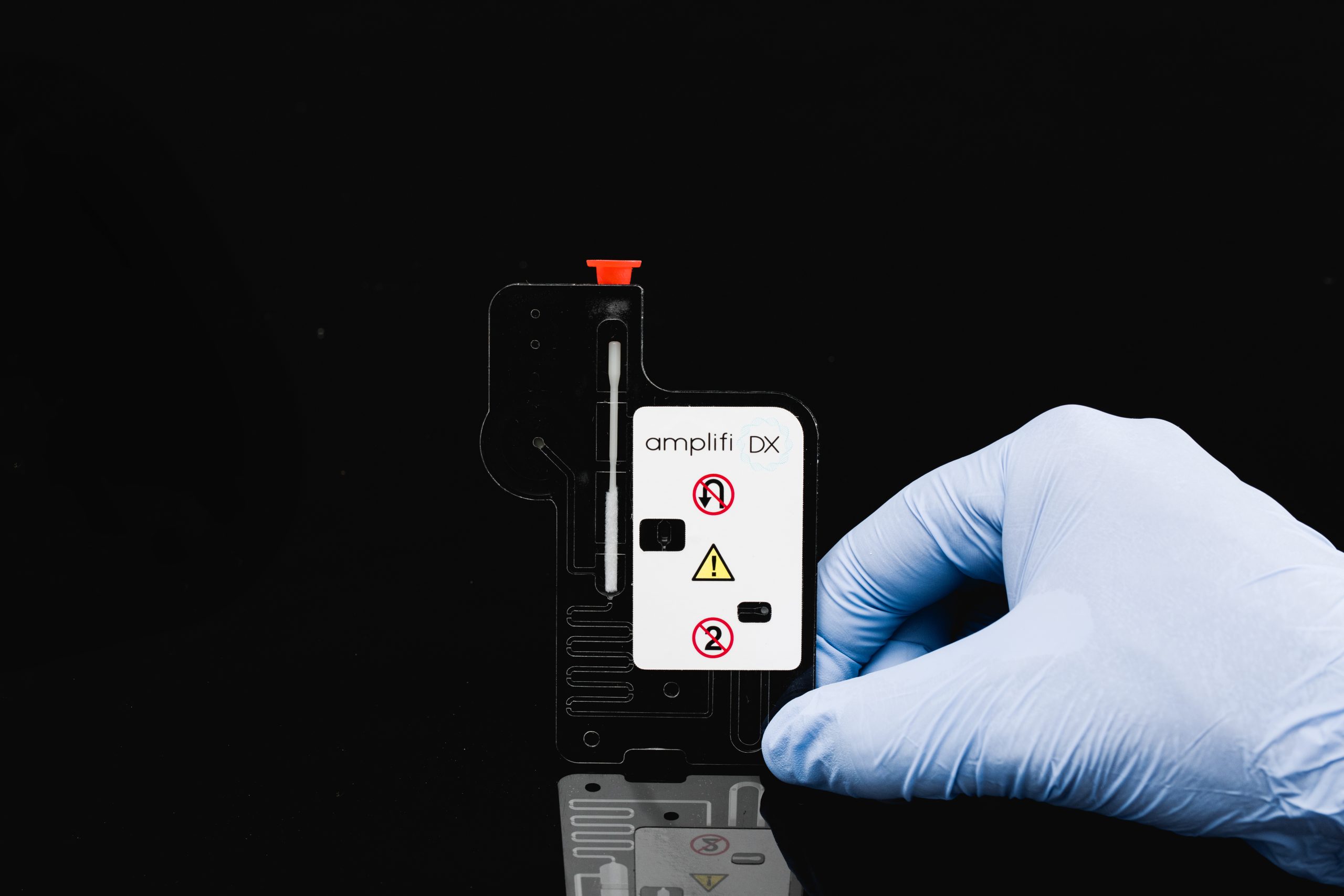
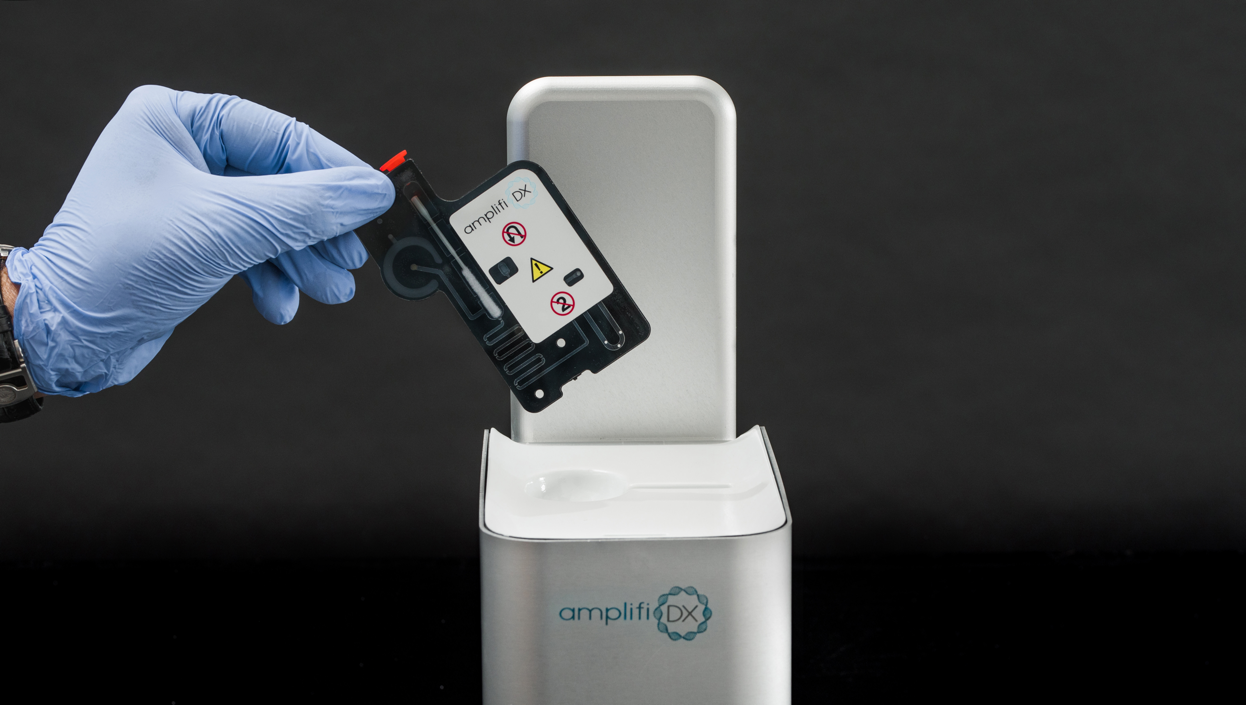
Swab to Answer Molecular Panel for Point of Care Diagnosis and Treatment
Delivering results within 30 minutes
AmplifiDX was awarded funding through the National Institute of Health Rapid Acceleration of Diagnostics (RADx) program to support the development of it’s point-of-care molecular testing instrument. The funds were used to develop the AmplifiDX DX-100 instrument and its RespiFast Assay, a nasal swab-based nucleic acid amplification test (NAAT) for COVID-19. Since then, they have also received NIH funding toward the development of an assay for point-of-care diagnosis for other respiratory and infectious diseases.
According to the NIH, the need to develop sensitive, specific, and more easily available point-of-care technologies for diagnosing sexually transmitted infections (STIs) is critical. Many countries, including the US, have seen a sharp increase in the incidence of STIs over the past half-decade, and especially since 2020.
Infectious diseases were responsible for 8% of deaths globally in 2022.

Project Phase 0
Feasibility
AmplifiDx received a RADx grant from the NIH to prove their concept in three months. Veranex was asked to start from scratch and build a proof-of-concept device to meet AmplifiDx’s phase 1 grant deliverable.
Project Phase 1
Design Inputs
Veranex worked with AmplifiDx to develop product requirements and helped set out a pathway for EUA approval through the FDA.

Project Phase 2
Design and Development
AmplifiDx came back to Veranex to complete the design and follow the FDA design control process for EUA submission.

Project Phase 3
Prototyping
Veranex developed five prototype units in order to have the systems tested and to be used for clinical trials.

Project Phase 4
Verification and Validation
Veranex tested the system, validated it to its requirements, and sent to AmplifiDx for use during clinical trials.

Project Phase 5
Transfer to Manufacturing
Veranex built five pre-production units as part of our bridge-to-transfer service, allowing AmplifiDX addional flexibility as they scale up.
INTEGRATED DESIGN & DEVELOPMENT EXPERTISE
Leveraging the pre-built technology infrastructure from Veranex, our team helped AmplifiDx hit an important funding milestone — design and fabricate a functioning prototyping in 90 days. Throughout the process, our team developed all components of the instrument, including industrial design, mechanics, electronics, software, firmware, and optics.
The team is now collaborating on new iterations of the instrument that will allow for broader testing capabilities.
“We believe through this continued partnership and with our innovative and accessible testing solutions, we’ll make a difference in people’s lives for years to come”
Nancy Schoenbrunner – CEO AmplifiDx



