Complete IVD Kits in Less Than a Year
Meeting the rapidly increasing demand for SARS-CoV-2 tests
Services
Industry
The Challenge
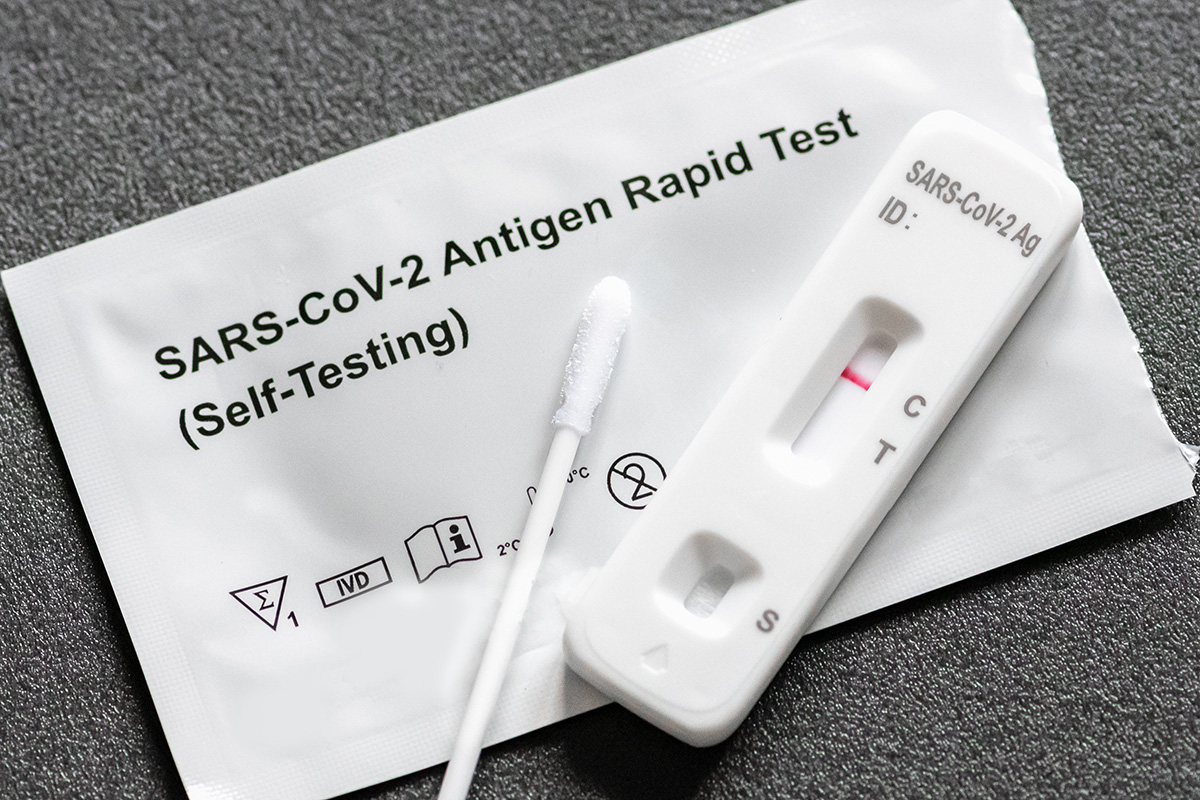
SARS-CoV-2 recently caused a global healthcare crisis. There was an increased demand for rapid diagnosis of SARS-CoV-2 due to long wait times for test results from overburdened or understaffed laboratories. The client’s vision was to design a novel paper-based immunoassay that could be administered by anyone with minimal training. Aiming for a fast and low-risk path to market, the client contacted Veranex for the development of an immunoassay in vitro diagnostic (IVD) kit.
The Solution
Veranex partnered with the client (2020-2021) and first reviewed the assay workflow in order to capture requirements that could impact specificity and sensitivity. Veranex worked closely with the client to improve test execution, interpretation of results, and to design for ease-of-use by minimally trained personnel. During the project, we provided expertise in assay development, translation and execution, mechanical engineering, regulatory guidance, program management, and selection of a high-volume manufacturer.


Project Phase 0
Immersion, Research, Innovation, Concepting
As part of the immersion strategy, Veranex brought testing in-house to understand the user requirements needed to execute the assay workflow. Robust and reproducible printing (by Veranex) of the consumable paper-based assay revealed an opportunity to improve the assay’s sensitivity. We leveraged our science team’s expertise and recommended reagent changes to the immunoassay kit that would not only improve the assay but would also facilitate high-volume printing. Learnings from this phase led to concepts for prototyping and testing of the diagnostic kit in phase one.

Project Phase 1
De-Risking, Feasibility, Design Vision
Veranex designed various product embodiments and used the client’s feedback as a guide to iterate prototypes. Through innovation at Veranex, the assay evolved to include a handheld device to support the dispense of microliter quantities of patient sample and included a novel printing scheme to guide fluid deposition onto the paper consumable. We also completed the design of the diagnostic kit to include the assay housing and test strip, a sample dispenser, test swab and sample tubes. We worked with the client to select a high-volume manufacturer and by the end of Phase 1, all designs and protocols were transferred to the third-party to manage the high-volume production of assay strips for research-based clinical testing and refinement of an app-based reader.
From Concept to Complete IVD Kit
A client needed a fully developed immunoassay IVD kit for SARS-CoV-2 detection. By working with Veranex, the client received guidance from our assay development and translation team, and our innovative engineering team. We distilled an intricate concept and developed it into a complete IVD kit in less than a year. We continued supporting the next developmental steps by careful selection of a large-scale manufacturer for further clinical research.
