Moderna
Rapid, Mobile Vaccine and Therapeutic Manufacturing
Deploying vaccines quickly via a transportable container

Services
The Challenge
of producing hundreds of doses of vaccines in a matter of days in a rapidly deployable 6-foot x 6-foot x 6-foot (1.8m x 1.8m x 1.8m) container.
The Solution
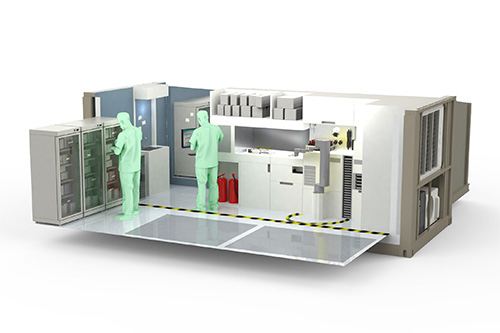
Project Phase 0
Immersion, Contextual Inquiry, Requirements Development, Concepting
We leveraged Moderna’s existing workstreams used in their mRNA vaccine platform as a starting point to assess the impacts to the target container size. Processes were mapped and high-level software and control architectures were developed. Veranex examined user needs and military-use environments to support the design and utility of the manufacturing unit. In collaboration with Moderna, the team performed technology reviews and identified opportunities for development, leading to bespoke concepts and alternative technologies that maintain quality-controlled vaccine production throughput. Aspirational design of the mobile unit and its subsystems was developed as a launching point for Phase 1 activities.
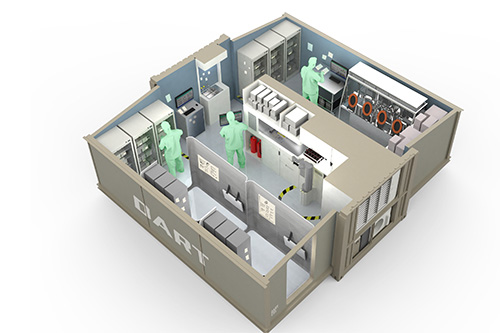
Project Phase 1
Subsystem De-Risking, Feasibility, Design Vision
Veranex moved forward with technical development and testing of alternative technology solutions to determine quality of results, ease of integration, and physical space claim within the mobile unit. We collaborated with Moderna to conceive, design, assemble, and test prototypes to de-risk critical steps in the vaccine production pathway. Consumables, reagents, and their storage requirements were also evaluated by Veranex to streamline packaging for on-unit storage for integration with the developing automated processes. Technologies that passed Moderna’s quality control specifications were selected for the Phase 2 activities.

Project Phase 2
Design Development & Design Outputs, Assembly
Veranex pre-assembled the modular frame to de-risk load capacity, frame tip-over and impacts to the frame by equipment vibration. After shipment to Moderna, both teams integrated the subsystems onto the unit for comprehensive testing of reagents, clean-in-place strategies, and precision performance with the selected robot handler. A strong collaboration allowed for the development and prototyping of additional solutions to improve efficiencies in system cleaning and quality control.
Realizing a Vision