← Product Design & Engineering
Engineering & Development
Our engineering and development services realize your product concept into a functional product using cutting-edge technology and years of healthcare industry expertise.
We follow ISO 13485 and FDA compliant processes and our own innovation methodology to develop and commercialize robust solutions. This enables customized products for your target market and user, providing a world class design experience that caters to your healthcare specialty.
Areas of proven development success include:
- Single Use Disposables
- Drug Delivery Combination Devices
- Capital Systems
- Surgical Instrumentation
- Surgical Robotics
- Infusion Pumps
- Catheter Design
- Endoscopic Devices
- Molecular Diagnostics (IVD) – capital and consumables

Applied Science
Understanding the scientific principles of your devices will drive the success of your product. Our applied scientists work with you and our engineers to transform complex challenges into novel innovations. Our applied science team is involved at the very start of programs to gain a thorough understanding of the disease state or physiology. Establishing and disseminating this knowledge is critical in the selection of technical solutions to meet your requirements. This approach is supported by targeted research, theoretical analysis and physical testing to guide decisions with data-driven and logical recommendations. We always take a practical and scaled approach – truly balancing the constraints of engineering, usability, manufacturing and cost. With expertise across the spectrum of scientific disciplines, our team of applied scientists can support your project by applying first principles to solve complex challenges.
Areas of expertise in applied science include:
- Microfluidics
- Optics
- Cellular and Molecular Biology
- Sensing Systems
- Advanced Analytics
- Biochemistry / Assay Development

Mechanical Engineering
Our experienced mechanical engineering team designs and develops medical devices from early concept phases onward. We use industry standard and state-of-the-art tools to model, analyze, test, and inform decisions to build confidence while reducing risk in the product throughout the development lifecycle. We constantly balance engineering analysis with empirical assessments to develop a foundational understanding of how your systems performs. Testing with real models, prototype and pilot devices give the real-world evidence to have confidence and iterate on your design. By considering the end goal from day one, our engineers create novel processes to complex mechanisms to deliver devices that are commercially and operationally viable.
Areas of expertise in mechanical engineering include:
- Complex part design
- Precision mechanisms
- Fluidics / microfluidics
- Reposable / reusable design
- Injection molded design
- Advanced analysis – Mold flow, comsol, fea, thermal analysis
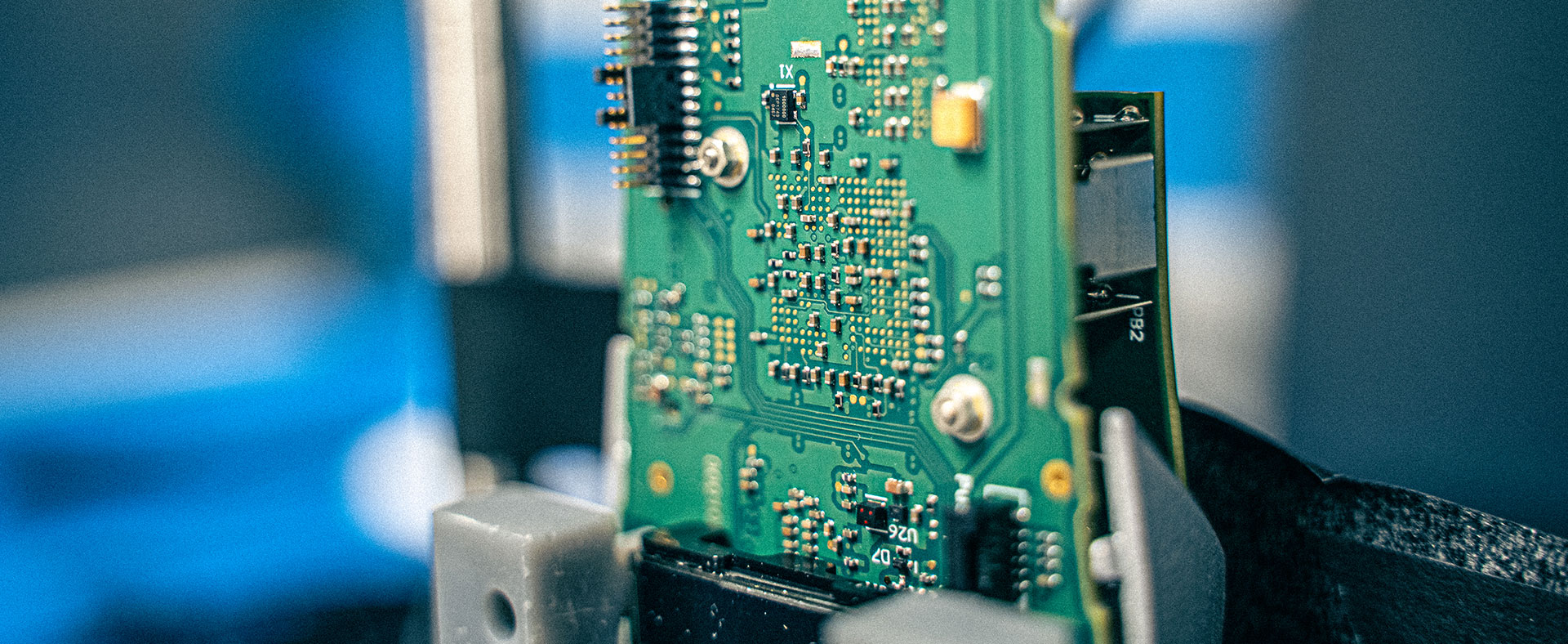
Electronic Systems
Our electrical engineering teams are highly experienced in developing electronics from low-power connected wearables to a complex point-of-care system. Our teams use a thorough understanding of regulations and requirements to realize your medical product. Our electronics labs are equipped to allow for rapid prototyping, testing and de-risking your product early in the development cycle. By working in tight collaboration with embedded software, mechanical and design teams, we strive for seamless integration of electronics into the medical device. We consider the regulatory pathway by taking a risk-based approach to deliver high-performing and compliant electronic systems.
Areas of expertise in electronic systems include:
- Electronics hardware design
- Control systems
- Power supplies
- Low power electronics
- Signal conditioning
- RF design
- EMC / EMI expertise including root cause investigation

Software Development Services
We have extensive experience providing expert solutions for healthcare companies that span varied therapeutic areas. From embedded firmware and software for safety-critical systems and complex real-time controls, to web and mobile applications and cloud computing, our team is passionate about delivering software engineering solutions with the highest quality.
Areas of expertise in Software Development
- Software in a Medical Device (SiMD)
- Embedded Software & Firmware
- GUI Applications
- Mobile Applications
- Web and Cloud Solutions
- UX/UI Design
- Software as a Medical Device (SaMD)
- IEC-62304, IEC 82304-1, and FDA Compliant
- Software Verification and Validation
- Cybersecurity
- LIS and EHR Integration
- Pre-developed platform technology

Testing & Compliance
With the complexity of products that exist in the medical technology space, quality and reliability cannot be compromised. Through our ISO 13485 regulated process and direct FDA experience, we embrace the rigor required but employ the flexibility to act within the regulations. Our team acts intentionally with a quality mindset from the beginning of programs providing input and direction with risk management and compliance testing in mind.
Areas of expertise in Testing & Compliance
- Certified to ISO 13485:2016
- Compliant to 21 CFR Part 820
- Design Controls
- DHF
- Risk Management
- FMEAs (Usability, Design, Process)
- Design Verification Testing
- Design Validation Testing
- Reliability Testing
- Product Safety Testing (IEC 60601-1)
- EMC Testing (IEC 60601-1-2)
- Emissions Pre-Screening
- IEC 62304 Medical Device Software
- Formative & Summative Usability Testing
- Environmental Testing
