← Product Design & Engineering
Manufacturing Solutions
We take pride in our world-class facilities, processes and capabilities and are dedicated to ensuring that your product meets the highest quality standards.
Offering tailored manufacturing capabilities to fit exactly what you need in terms of scale and timing- there is no build too small – we do not have minimum volume requirements. This means we can scale with you as you grow your business. The knowledge that initial builds can make or break a budget during your market release plan is a consideration that for every build that happens at our facility.
Through verification and validation to human use, commercial builds we have the processes and facilities to manufacture your product into reality.
Process Validation, Manufacturing Transfer, Design Validation
Commercialization-ready design
Move your product toward launch with our world-class project, manufacturing, and quality teams. Our engineers oversee production, tooling development, and configuring manufacturing lines, followed by process validation, risk management, and failure modes and effects analysis (FMEA). From building and testing pilot units to assembly and design validation studies, we prepare and refine until it’s time for submission for your product.
Services:
- Process validation
- Production-ready lines
- Risk management activities
- Complaint Investigations
- Design validation testing
- Regulatory submission and support
- Clinical Study Builds with fully traceable material

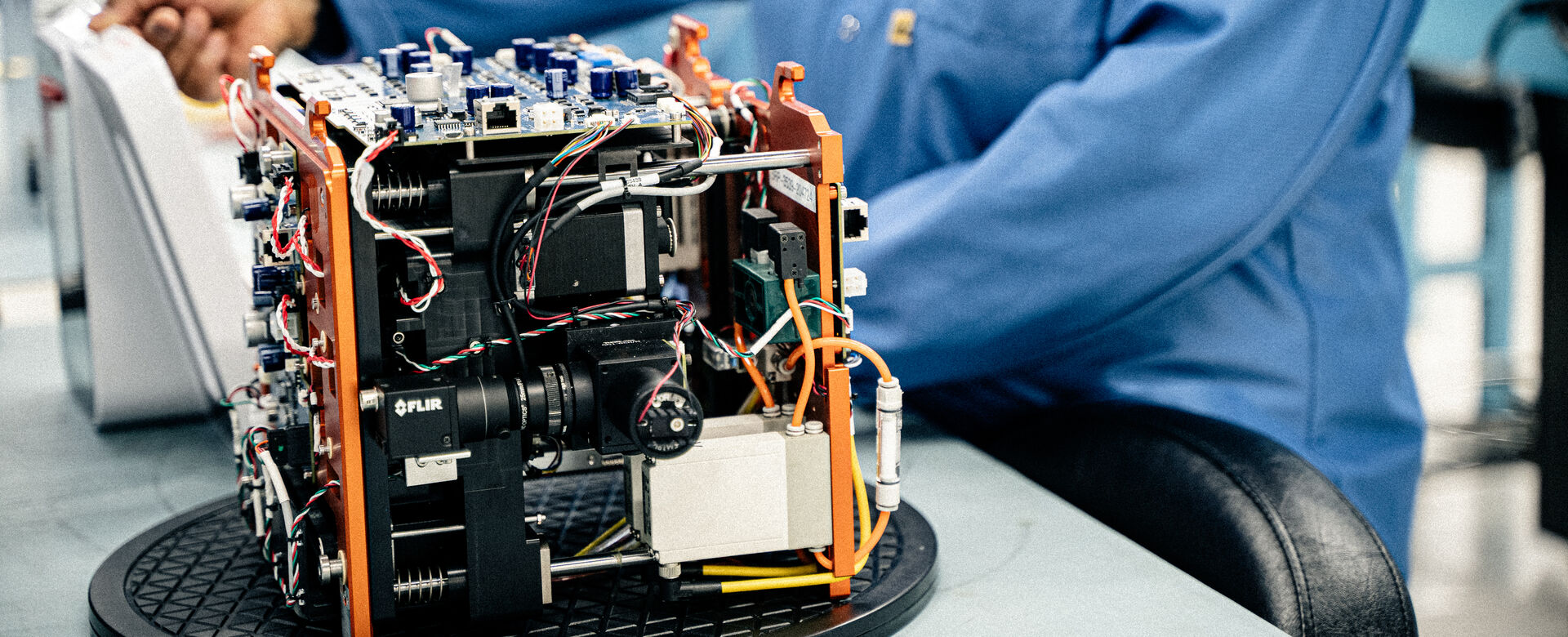
Contract Manufacturing and Integrated Supply Chain
Capabilities
We offer turnkey capabilities for manufacturing quality-controlled builds that can support clinical through human use volumes to meet your needs as your market share increases. We can develop your Supply Chain by leveraging our robust AVL(approved vendor list) and international footprint to make sure your program has what it needs, when it’s needed, every time. If and when the time comes to move to another partner, we will support your transfer seamlessly without any continuity disruption.
Services:
- Supply chain strategies, planning, development, and management
- Supplier Audit Support and Supplier Management Program
- Product Cost Analysis – COGs (by region as needed)
- Clean Room Production & Assembly (Class 7 & 8)
- Sterilization management
- Part inspection and controls
- Commercial launch and lot release
- Device Investigation Support
- COGS analysis
- End of Life & Obsolescence
- Depot Service Repair

Facilities
The Manufacturing Team employs a talented and diverse team of Engineers and Specialists. We work closely with the research, human factors, design, quality, and engineering teams to deliver innovative and robust manufacturing and supply solutions. A comprehensive, yet flexible, tried and true QMS along with up-to-date versions of tools such as MRP, ePDM, and CAD give our employees the framework and capacity to get you the results you need.
Unique Qualities:
- FDA Approved Manufacturer of Medical Devices
- ISO 13485-2016 Certified
- ESD-Controlled Manufacturing Area
- 100K & 10K Clean Room(s) ISO Class 7 and 8
- Dedicated/Flexible Manufacturing Space
- Component/Finished Goods Inventory Management