TAVI
Improving TAVI Performance for Transcatheter Aortic Valve Replacement
The path to becoming the leading market competitor
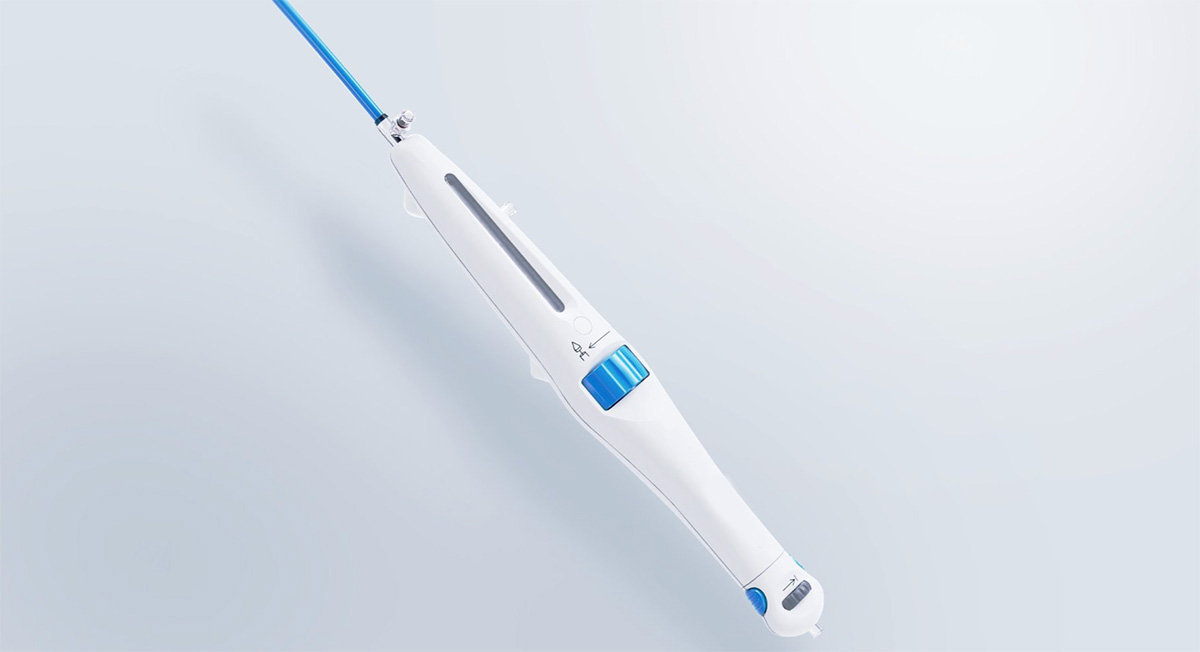
Services
Industry
The Challenge
In response to evolving market demands due to the increasing prevalence of aortic stenosis disorder, the client was determined to improve their transcatheter aortic valve implantation device (TAVI) to become the preferred standard by surgeons. The client partnered with Veranex in the redesign and upgrade of their existing TAVI device for transcatheter aortic valve replacement (TAVR).
The Solution
We examined how the current device performed, and identified and addressed unmet needs to improve the overall experience when performing this procedure. The result was drastically improved ergonomics in use and the creation of a new product experience to deliver best-in-class solutions.
Project Description
Our team conducted contextual inquiry activities which helped us identify preliminary user needs and discover insights that guided concept development for the next-gen handle. This was followed with cross-disciplinary concepting in which our teams down-selected to five distinguished directions. We generated two rapid prototypes and tested within a wet lab to verify force requirements, then conducted a formative study and usability testing — ultimately landing on a single concept design that mitigates the most common points of friction and fulfilling identified areas of opportunity. The process took place over 16 months.
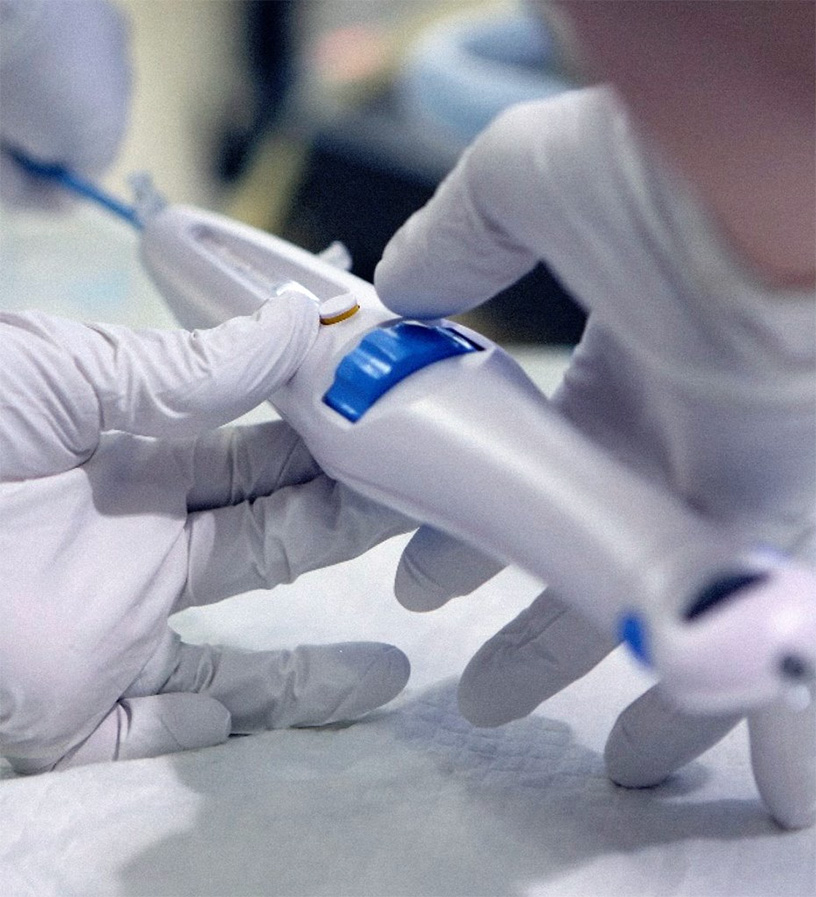
Condition Description
Aortic valve stenosis is a type of heart valve disease where the valve between the lower left heart chamber and the body’s main artery is narrowed and doesn’t fully open. It results in reduced or blocked blood flow from the heart to the rest of the body which can lead to death.1 It’s the second most common valvular lesion in the US and is present in about 5% of the population at age 65 with increasing prevalence with advancing age.2 To reduce the symptoms of aortic valve stenosis, doctors can perform a minimally invasive TAVR procedure, in which a TAVI device is used to guide a thin, flexible catheter to the heart through the blood vessels. Inside the catheter is a replacement valve, which is placed securely within the old valve to allow blood flow. Given the competition within the field of TAVI devices and the prevalence of this procedure, it is critically important to continually improve usability of the device to provide physicians with the smoothest possible experience.
References
1 https://www.mayoclinic.org/diseases-conditions/aortic-stenosis/symptoms-causes/syc-20353139
2 https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-18/epidemiology-of-aortic-valve-stenosis-as-and-of-aortic-valve-incompetence ai#:~:text=Aortic%20stenosis%20(AS)%20is%2C,increasing%20prevalence%20with%20advancing%20age.
Project Phases

Phase 1: Discovery
Understanding Users
Our team conducted contextual inquiry activities to fully immerse ourselves in the existing product, its users, and use environments. This work helped us identify preliminary user needs and discover insights that guided concept development for the next generation handle design.
We spoke to five key opinion leaders, conducting in-depth interviews and site observations across three global regions. These interviews helped us uncover not only the needs of surgeons familiar with the current system, but the needs of their support staff as well.

Phase 2: Design Exploration
Cross-Disciplinary Concepting & Applying Insights to Design
Our learnings in the immersion process guided us through a series of cross-disciplinary concept ideation workshops made up of researchers, industrial designers, mechanical engineers, and human factors engineers from both our team and the client's. In bringing together a diverse array of industry experts, together we generated over 175 initial ideas, carefully down-selecting to just five compelling directions against a highly considered criteria.
Using outputs from the co-ideation workshops, our team created distinct handle designs from the five original concept directions, each of which incorporated valuable insights around ergonomics, usability, and aesthetics.

Phase 3: Engineering Builds
Emulating Experience & Testing for Success
Rapid prototype creation enabled our team to engage in both early mechanical development and preliminary benchtop testing of our design concepts. By testing our prototypes within a wet lab, we verified that the mechanical solutions met force requirements for catheter deployment.
Creating a mechanism in CAD isn’t enough. Ensuring surgeons can intuitively and reliably navigate a catheter requires testing. We looked to our working prototypes to validate whether or not the chosen valve deployment controls could adequately meet force requirements from manual actuation effectively and comfortably.
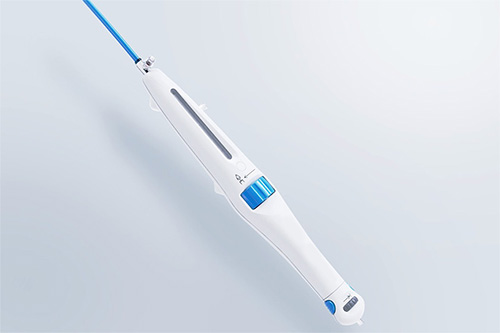
Phase 4: Usability Testing
Formative Testing for Final Adjustments
Maintaining the user at the centerof the project, our human factors team put a “looks-like” prototype of concept 2 and concept 4 in the hands of several surgeons to evaluate their needs and preferences for critical handle features, such as the deployment wheel, central lumen pullback, and deployment lock.
Success
Using all of what we learned so far — from early discovery through IDIs, design exploration through co-ideation workshops, and design refinement through mechanical testing, formative study, and usability testing — we landed on a single design concept, carefully honing it to mitigate some of the most common points of friction and identified areas of opportunity.
The Outcome
Familiarity
To allow minimal disruption to the existing workflow, we improved features such as co-axial wheel deployment to enable intuitive usability.
Balance
We intentionally placed weight at the centre of the handle to better equip the user with improved precision and control.
Accessible Form
To encourage adoption from a diverse array of surgeons, we selected a tapered handle form better suited for hands of all shapes and sizes to naturally and comfortably access controls.
Undivided Attention
We designed the product’s controls to ensure surgeons know exactly how to deploy the valve, even when their eyes are on a monitor, not their hands.
