Thrombectomy System
Treating a Time-Sensitive Disease State
Creating a new thrombectomy system for improved patient outcomes.

Services
Industry
The Challenge
The Solution
Condition Description
Acute limb ischemia (ALI) is a sudden and rapid decrease of perfusion that threatens the viability of the limb. ALI is often caused by acute thromboses of a peripheral artery where a clot has blocked the blood vessel and prevents blood flow. If blood flow isn’t restored quickly, patients can suffer severe damage; the worst cases can result in limb loss or death.
Acute Limb Ischemia has high morbidity and mortality rates. One in five people die and 30% of patients will lose a limb. Irreparable damage can occur within 4–6 hours of onset, but the average time to intervention is 10.2 hours.

Project Phase 1 Sprint-to-Market
Rapid Ideation, User Testing, & Mechanism Exploration
Our team created feasible conceptual solutions for various workflows, user interactions, and device forms. This enabled us to create holistic system architectures with proposed visual designs and mechanisms. Using 3D-printed prototypes, we evaluated the early esigns with key opinion leaders on usability and ergonomics. In parallel with ID, our engineering team explored several mechanisms for the wire lock feature, which ensures the user can capture and extract the thrombus.
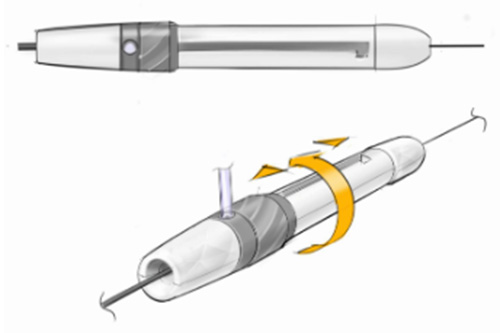
Project Phase 2 Design Refinement
Enhancing Speed and Efficiency Without Compromising on Ergonomics
In this phase, we worked with the client to create a unique form language to establish the thrombectomy device’s brand. We made improvements in the basket slider that allowed for two- or one-handed activation, made the user interface more intuitive, and considered touchpoints to prioritize ergonomics.

Project Phase 3 Design for Manufacturing
Integrating Design & Engineering
Our designers and engineers collaborated from concept sketches to the final build to ensure that the design intent fulfilled user needs, the client’s brand vision, and be technically sound for manufacturing. This partnership enabled the engineering team to quickly translate the final concept into a tooling-ready design.
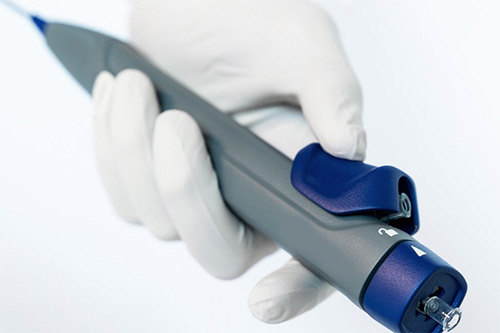
Project Phase 4 Visual Form Language
Refining the Brand System
Veranex developed a set of iconic visual elements that make up the look and feel of the thrombectomy device’s brand. These details can be applied systematically to new products to speed up the design process and establish a unified visual family.
Success
With help from Veranex, the client was able to go from a prototype to manufactured parts in a matter of months. In 2023, the system received its FDA 510(k) clearance. We worked with the client to establish an ownable industrial design brand language for the thrombectomy system that can be applied to the rest of the products within the portfolio. With input from users and key opinion leaders, we developed and implemented a simple, intuitive interface that maximizes safety and usability.
