6 min read
The Strategic Science of Optimal Preclinical Model Determination, Selection and Development
 Nicolas Borenstein
:
Aug 5, 2025 11:41:08 AM
Nicolas Borenstein
:
Aug 5, 2025 11:41:08 AM
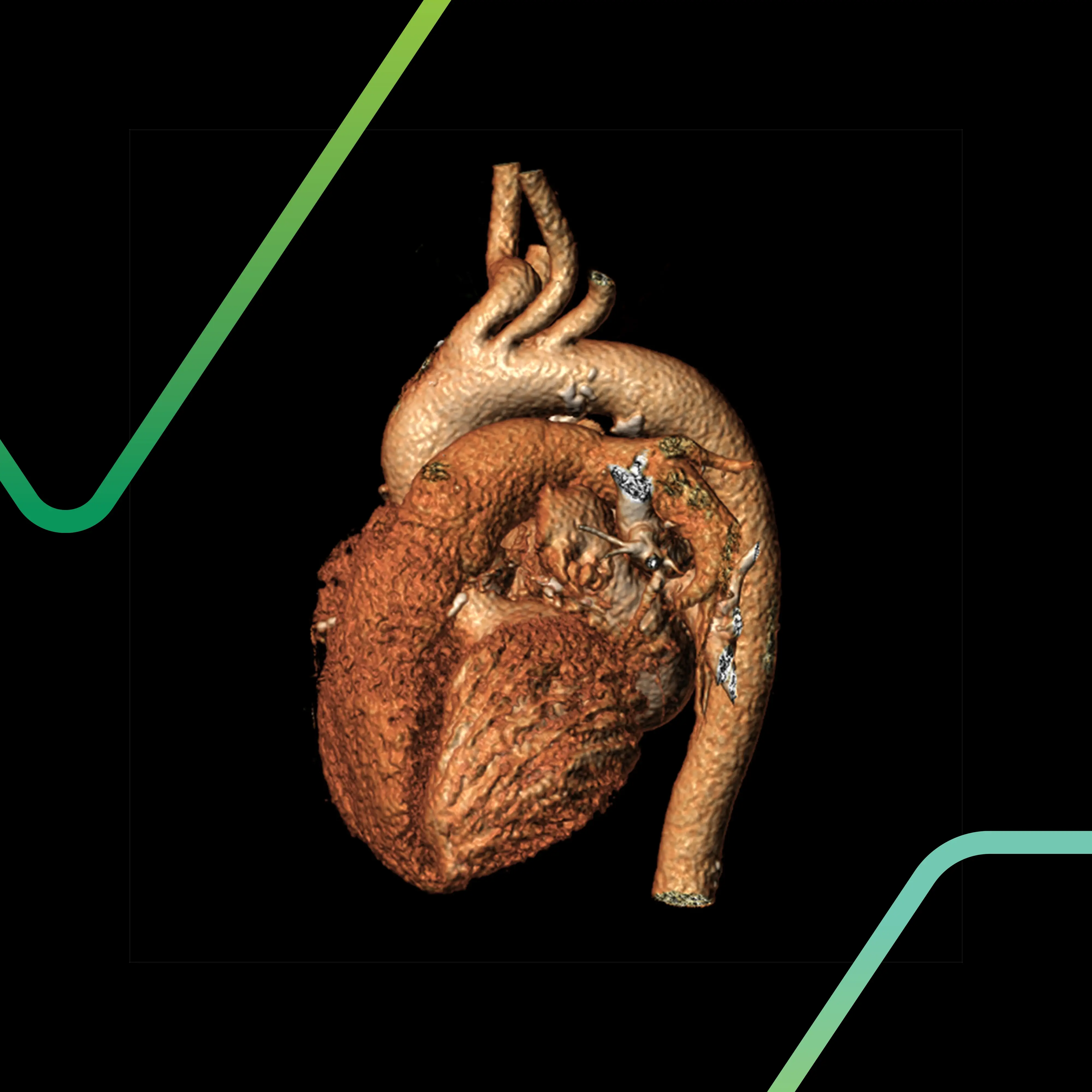
Don't ask "Can this model work?" Ask "Is this the optimal preclinical model for regulatory success?"
When your breakthrough, truly novel or generational new medical device idea transforms from napkin sketch to regulatory submission, one critical decision can determine whether you fast-track to market or face costly delays: choosing the right preclinical model.
It sounds straightforward until the wrong model leads to repeat studies, regulatory setbacks, and hundreds of thousands in added costs. But when chosen strategically, the right model becomes a competitive advantage, streamlining your path to market.
In preclinical research and medical device testing, success hinges on finding a preclinical model that strikes the right balance between scientific rigor and operational feasibility. You need a model that mimics human anatomy and physiology just enough, without adding unnecessary complexity, cost, or time.
[Contributors: Nicolas Borenstein (DVM, MsC, PhD), Jennifer Gordon, Michael Sweet]
Why Smart Preclinical Model Selection Matters More Than Ever
An optimal preclinical model isn’t just about biological similarity. It’s also about cost-efficiency in terms of acquisition, housing, husbandry, and regulatory strength. In GLP studies especially, it’s critical that the model supports safety and performance claims that can withstand scrutiny.
Your preclinical partner should have the experience and precision to help you get this right. For example, if 12 subjects are scientifically sufficient, don’t waste resources running 20 – or risk falling short by enrolling 10. Precision matters.
Beyond "Close Enough": Why Anatomic Equivalence Can't Be Compromised in Some Preclinical Testing
For interventional devices, particularly in cardiovascular or neurovascular applications, preclinical model anatomic fidelity isn't negotiable. “Close enough” isn’t good enough. When you're developing a TAVR system, for example, what matters is the aortic anatomy, ascending aorta length and shape, height of the coronary ostia, etc. When you're creating a neurovascular access device, cranial volume and vessel diameter are non-negotiable. You’re not just testing a device; you’re testing how it behaves within a living, dynamic system.
“This is why equivalence in aortic diameter, vessel wall thickness, or resection margins is critical,” explains Michael Sweet, Director of Preclinical Study Management at Veranex PCS. “It’s about how the device navigates, how much force it applies, and how it performs long-term.”
The stakes are real. Even small anatomical differences can derail a study. A device designed for a 0.5 to 0.7ml delivery volume, for instance, may perform flawlessly in one model but fail in another, even if that model is anatomically "close enough" in other respects.

The Behavior Factor: How Animal Temperament Affects Study Integrity
Animal temperament is a critical variable that can directly affect surgical outcomes, implant retention, catheter patency, and data integrity in survival studies.
For instance, young goats and smaller sheep tend to be active and restless, increasing risks to wound healing, post-operative implant retention, and telemetered data collection. In contrast, Yucatan swine or adult ewes tend to be more docile, improving conditions for catheter stability, post-op recovery, and cleaner data sets.
These behavioral traits should be factored into non-GLP model confirmation studies, where selecting the wrong species or age group can lead to setbacks, added costs, or even major protocol rework. Getting it wrong here, you risk delays, rework, and significant added costs.
When Flexibility Disappears: Navigating the Hard Limits of Preclinical Model Selection
In many high-precision preclinical studies, you don’t have room to compromise. Preclinical model selection becomes inflexible when device size cannot be scaled to accommodate the animal model, and device preparation/deployment must mirror that of the intended clinical workflow. Here’s where preclinical selection becomes mission-critical:
Structural heart procedures (TAVR, TMVR) and large-bore vascular devices: Most swine and ovine models hit anatomical limits in vessel diameter, max out in vessel size, leaving little flexibility. There’s no margin for models that don’t match procedural demands. Therefore, preoperative planning and animal screening is paramount.
Neurovascular access: Only specific breeds offer the vessel size and structure needed for carotid or femoral navigation without modification, and vessel diameter constraints eliminate most options.
Long-term degradation or immunological interaction studies: Species-specific responses dictate what's scientifically feasible – there’s no shortcut when biology sets the rules.
When flexibility vanishes, your preclinical partner must be ready. That means having the experience, facilities, and protocols – and knowing how to scale up to large animals or specialized endpoints. Otherwise, you face the high cost of starting over.
When Large Preclinical Models Are Unavoidable
Sometimes, large preclinical models like bovine or adult ovine are non-negotiable. When that’s the case, your preclinical CRO must have the facilities, expertise, and regulatory compliance to handle them properly - meeting USDA and GLP regulations while ensuring high interventional fidelity that replicates human surgical workflow.
This isn’t just operational – it’s a marker of your CRO’s commitment to surgical excellence, data integrity, and regulatory readiness. Facilities with AAALAC accreditation, while not mandated by the FDA, demonstrate a commitment to excellence including proper animal care protocols, reducing your regulatory risk all the while ensuring the utmost respect is given for animal welfare.
What Sets Apart True Interventional Expertise
At Veranex, our industry leading preclinical contract research labs in Atlanta and Paris specialize in the unique challenges faced by MedTech innovators. Our world-class team excels in structural heart, peripheral vascular, EP, neurology, orthopedics, ophthalmology, regenerative medicine, and wound healing.
We bring:
- Procedural expertise in cardiac and neuro interventions, including long-term implant and device function studies.
- Large-animal models of chronic heart failure and neurovascular pathology, supporting high-fidelity, translational outcomes.
- Advanced imaging, navigation, and hemodynamic monitoring tools that enable precise deployment and functional assessment.
Unlike preclinical CROs who retrofit general large-animal setups, our models are specially refined through continuous collaboration with surgeons, advanced imaging modalities, and real-world device constraints. We also develop new models where no predicates exist – de-risking device development in uncharted areas.
We provide end-to-end study support, from pre-GLP study design and pre-submissions, through 510(k), IDE, De Novo and PMA submissions with hybrid catheter/surgical environments and deep device experience across our multidisciplinary teams.
The Critical Questions Every Innovator Should Ask
Before selecting a preclinical CRO, demand specific answers to these key questions:
What preclinical model do you recommend and why? Ensure they can explain the anatomical and physiological comparability in detail.
Have you used this model for similar devices? Experience with your device category matters.
Can you support this model through long-term survival or degradation studies? Some CRO’s can’t handle chronic endpoints.
What's your recovery and post-operative management plan? Look for depth and specificity.
Who conducts your histopathology? Pathology isn’t just reading slides – it’s providing insights that accelerate timelines and reduce risk. Tenured veterinary pathologists understand the end-to-end challenges of medical device development and commercialization. The competitive advantage lies in a preclinical CRO partner that offers full-service pathology seamlessly integrated with testing facility operations - optimizing strategy, budget, and timelines.
The competitive advantages histopathology labs embedded in preclinical research facilities for medical device innovators: seamless communication, protocol optimization, enhanced efficiency, and superior quality control can mean the difference between regulatory success and costly delays.
The most effective approach combines traditional CRO capabilities with comprehensive innovation expertise including device research, design and development, software development, human factors, and manufacturing.
The result is pathology data that doesn't just meet regulatory requirements, but actively supports broader innovation goals and commercialization timelines, ensuring every study contributes strategically to your device's successful journey from concept through commercialization.
Red Flags to Watch for in CRO Recommendations
Be cautious if your CRO:
- Lacks experience with your specific procedure or device type. Innovators should heavily weigh studies done in the model and therapeutic area when evaluating the quality of a prospective preclinical partner.
- Underestimates procedural or recovery complexity.
- Claims “equivalence” without published validation or prior work.
- Lacks imaging or surgical capabilities matching your real-world procedure.
These gaps can compromise data quality, validity, and increase the risk of regulatory delay.
The Veranex Difference: Where Science Meets Strategy
At Veranex, quality starts at the design phase, not after your device is built. Our 130+ preclinical professionals, including 20+ veterinary surgeons across North America and Europe deliver the complex evidence that regulators, investors, and clinicians trust. We perform over 3,000 procedures annually, with devices now benefiting more than 1 million patients. We don’t just test your device – we help accelerate your path to market with confidence.
The Bottom Line
Choosing the right CRO for preclinical testing, especially GLP testing, can be the difference between market success and costly setbacks. Pick a partner who understands both science and strategy including an optimal preclinical model – one who validates their expertise with evidence, not just claims.
Learn more about how Veranex's Preclinical Services and Pathology Services can accelerate your medical device development timeline while ensuring regulatory success from the start. Contact us today to discover how our approach and comprehensive human factors and regulatory teams’ expertise can turn your next FDA pre-submission meeting into a competitive advantage.
About the authors:
Nicolas Borenstein, DVM, PhD, serves as co-president of preclinical services, with extensive expertise in surgical and transcatheter preclinical science, particularly in cardiovascular and non-cardiovascular medical devices. A widely published author and peer-review journal reviewer, Nicolas combines academic excellence with hands-on surgical experience. He completed his veterinary medical and surgical training in Paris and Fort Collins, CO, earned his MSc in Surgical Science under Professor Alain Carpentier at Broussais Hospital, and received his PhD summa cum laude from University Paris Cité. His additional qualifications include specialized certifications in microsurgery, experimental surgery, and animal research from prestigious French institutions.
Jennifer Gordon is Director of Quality Assurance for Veranex Preclinical Services. She is responsible for leading QAU and ensuring compliance with GLP, OECD GLP, USDA, and AAALAC. 16+ years of program management, process improvement, and Quality Systems experience.
Michael Sweet is the director of preclinical study management and a study director at Veranex Preclinical Services – Atlanta, bringing 15 years of specialized expertise in GLP-compliant preclinical research. His extensive experience spans ECMO/VAD devices, interventional cardiovascular procedures, and structural heart models, establishing him as a leading authority in complex medical device evaluation. As a biomedical engineer and researcher, Michael integrates deep technical knowledge with hands-on expertise across multiple imaging modalities, including ultrasound, micro-CT, MRI, and CT. His proficiency in scientific data analysis, advanced imaging technologies, and cardiovascular study design, combined with his ability to translate complex findings into clear, actionable scientific documentation, makes him an invaluable asset to medical device developers seeking rigorous preclinical validation of their innovations.
Preclinical Services at Veranex generates the evidence that clears the way for first-in-human studies, especially in complex cardiovascular and structural heart devices where Veranex is recognized as a leader. GLP and non-GLP studies are designed by device-savvy veterinary surgeons with fast access to Product Design & Engineering, Manufacturing Solutions, Data Analysis and Biocompatibility teams to ensure models, procedures, and endpoints reflect clinical reality. In-house Pathology & Histology delivers integrated interpretations, while Regulatory Affairs, Quality Consulting, and Clinical Research teams use these findings to shape submissions and trial designs. The result is fewer regulatory questions and a faster path into the clinic.




