Actionable Clinical Trial Data Visualizations
Drive informed decisions with clear, cost-effective insights into study progress and risk, powered by our expert clinical trial dashboard solutions.
Visualize Smarter, Decide Faster: Leverage Veranex Clinical Trial Dashboards for Medtech Insights
In the fast-paced world of Medtech trials, leveraging your data for rapid, actionable insights is crucial. This is where Veranex’s advanced clinical trial data visualizations excel. Our intuitive study progress dashboards are specifically designed to transform complex information into clear, interactive displays, enabling your team to visualize smarter, decide faster, and act with confidence.
See the Difference with our Expertise
-
Enhanced Decision-Making
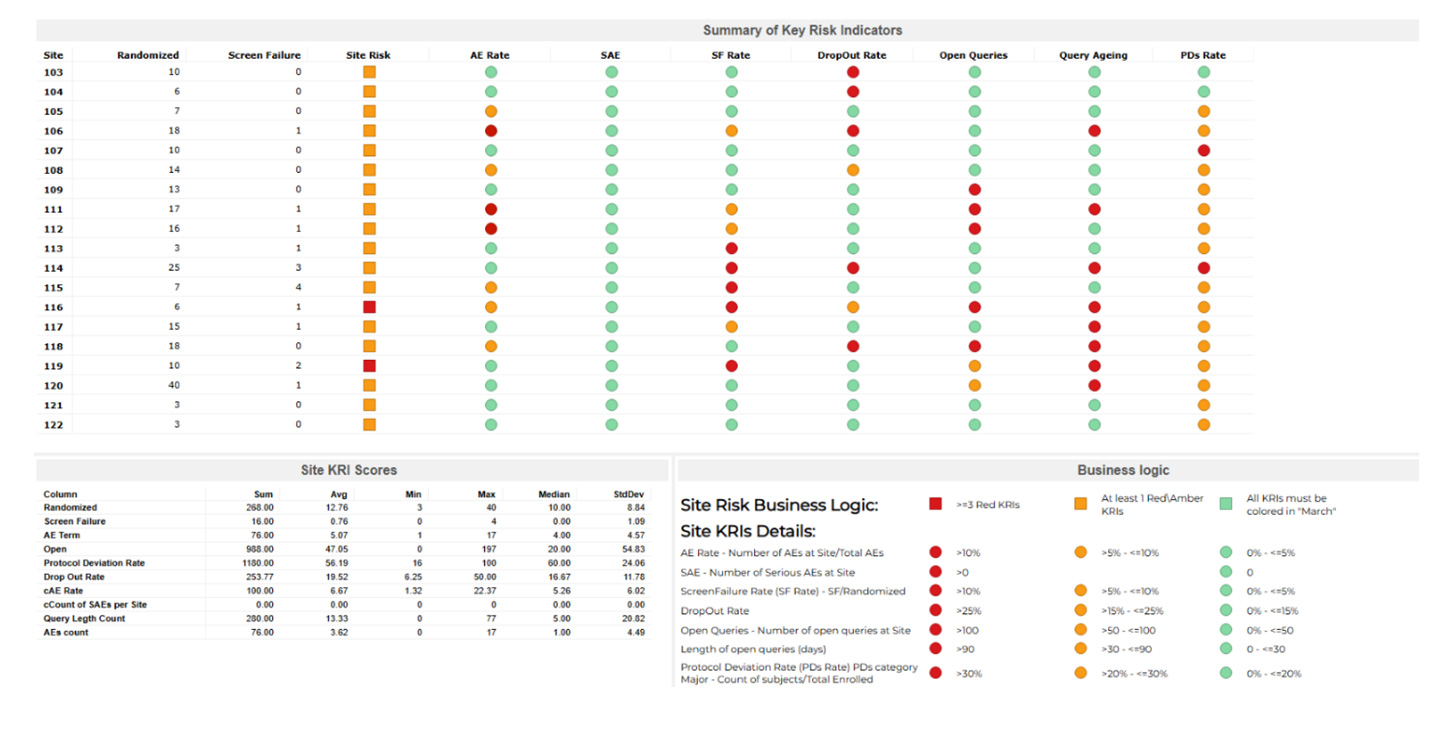
Our tailored Key Risk Indicator (KRI) dashboards provide real-time insights, allowing you to identify and quantify risks promptly. This proactive approach helps prevent trial disruptions and ensures compliance with regulatory standards. By visualizing critical metrics such as screen failure rates, adverse events (AEs), serious adverse events (SAEs), and query rates, you can detect potential issues early and take corrective actions before they escalate.
-
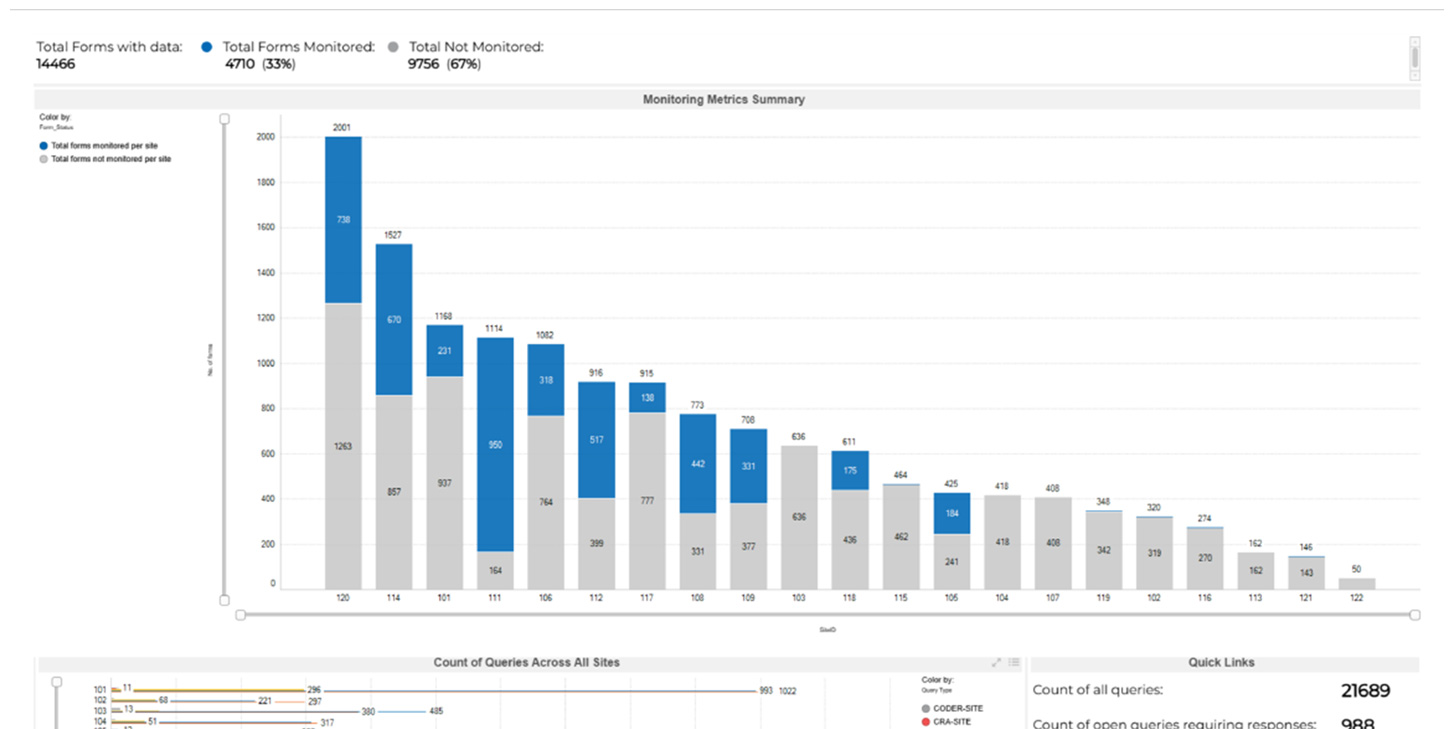
Comprehensive Site Metrics Monitoring
The performance and compliance of each clinical trial site is essential for maintaining data quality, patient safety, and operational efficiency. Veranex's site metrics trackers offer a detailed view of site activities, helping you ensure that all sites adhere to database lock timelines and other critical milestones. This level of oversight is particularly beneficial under risk-based monitoring frameworks, where timely and effective clinical monitoring is paramount.
-
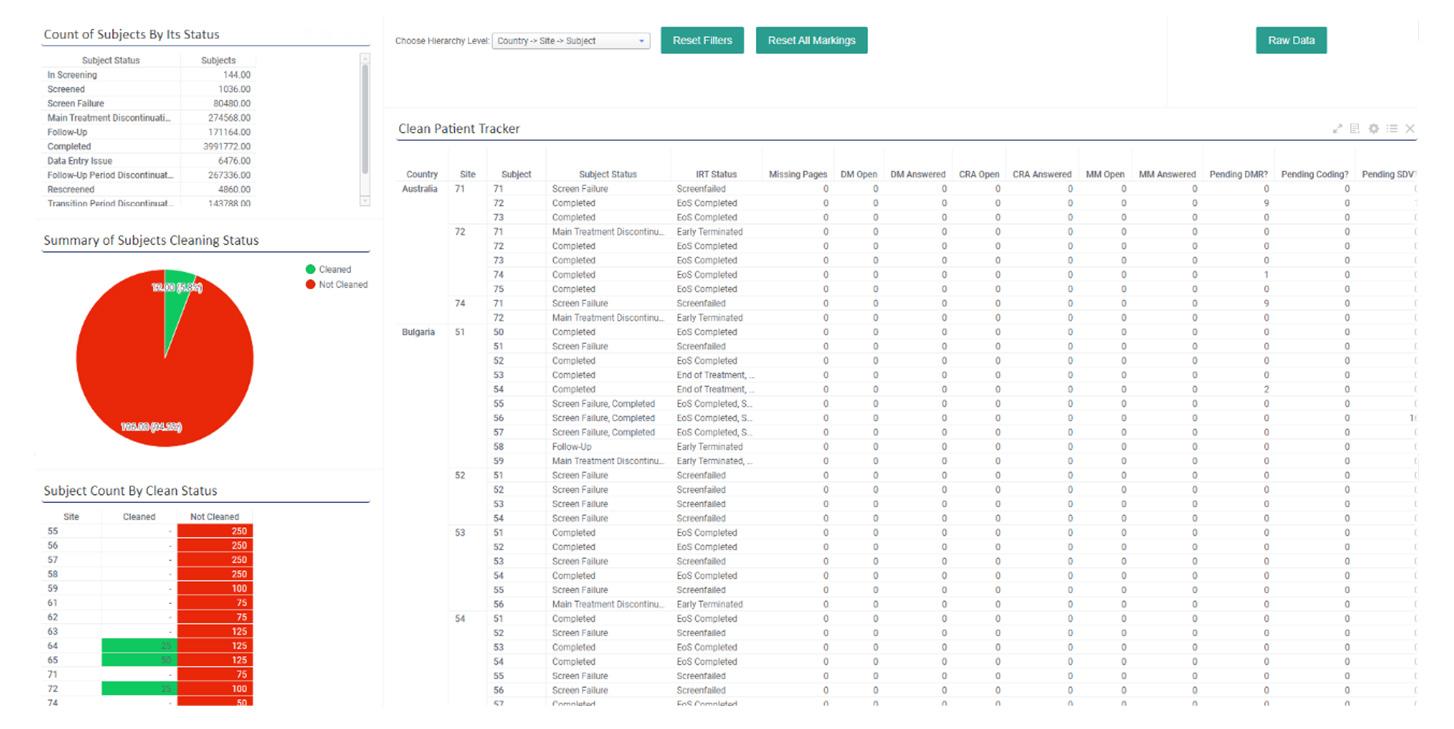
Clean Patient Tracker (CPT)
Ensure data readiness for analysis and accelerates database lock timelines. By prioritizing sites that are lagging in queries and source data verification (SDV) issues, the CPT helps streamline your trial processes and enhances overall efficiency. This tool is invaluable for keeping your trial on track and ensuring that your data is always ready for analysis.
-
Global Talent and Expertise
At Veranex, we leverage a global pool of talent and advanced analytics to provide you with the best data visualization solutions. Our team of experts is well-versed in medtech trials and understands the unique challenges and requirements of the industry. This expertise ensures that our visualizations not only meet regulatory requirements but also maintain the integrity of your study, fostering trust and confidence in your results.
-
Sophisticated Alerts
Safer Trials Safety is a top priority in medical device trials. Veranex's high-end sophisticated alerts for adverse events (AEs) and serious adverse events (SAEs) ensure that you are always aware of any potential safety issues. These alerts enable you to take immediate action, ensuring the highest standards of patient safety and trial quality.
-
End-to-End Dashboard Development
We offer comprehensive development of clinical trial dashboards, from initial design to final implementation. Our end-to-end approach ensures that your dashboards are tailored to your specific needs and provide the insights you need to drive impactful results. By using reusable templates, we can deliver dashboards quickly and efficiently, saving you time and resources.
Data Visualization That Delivers 28% Faster Study Insights
Effective clinical trial data visualizations can significantly reduce the time needed to understand study status by as much as 28% (Tong et al., AMIA Annu Symp Proc. 2017). Veranex empowers you to achieve this efficiency with our in-house visualization tools, refined through direct client feedback.

Stay Ahead with Innovative Clinical Trial Data Visualization Solutions
Interactive Dashboards
Enable faster decision-making with real-time insights. Our interactive dashboards provide a clear and comprehensive view of your data, allowing you to make informed decisions quickly.
Site Metrics Trackers
Ensure adherence to database lock timelines for seamless operations. Our site metrics trackers help you monitor and manage site performance, ensuring that your trials stay on track and meet critical deadlines.
Drill-Down Capabilities
Gain deeper insights into your data for more informed choices. Our drill-down capabilities allow you to explore your data in detail, uncovering trends and patterns that can inform your strategy and improve outcomes.
Faster Dashboard Delivery
Benefit from quicker development using reusable templates for efficient reporting. Our approach to dashboard delivery ensures that you get the information you need promptly, with templates that streamline the reporting process and enhance efficiency.
Transforming Clinical Trials with Real-Time Data Visibility and Actionable Insights
At Veranex, we convert static reports into clear, actionable visuals that accelerate decision-making. Our real-time dashboards provide critical trial visibility, enabling swift, confident decisions while ensuring regulatory compliance.
Our proprietary visualization technology integrates with multiple clinical applications, centralizing data in one unified location. We deliver cost-effective solutions or seamlessly adapt to your existing platforms (Tableau, Spotfire, SAS, R Studio), minimizing learning curves while maximizing insights that drive informed decisions and accelerate success.
-
Data Wrangling CapabilitiesSeamlessly combine data from various sources for a holistic view of your trial.
-
End-to-End Dashboard Development
We create comprehensive dashboards tailored to your needs, featuring both standard and customized visuals.
-
Dedicated Clean Patient Trackers (CPTs)
Receive customized CPTs designed specifically for your individual studies to track patient data effectively.
-
KRI Trackers
Monitor site performance with our Key Risk Indicator trackers to ensure compliance and efficiency.
-
Sophisticated AE and SAE Alerts
Enhance trial safety with advanced alerts for adverse events (AEs) and serious adverse events (SAEs), ensuring proactive risk management.
-
Statistical Modeling
Utilize robust statistical modeling to evaluate critical data and identify trends and patterns through various visualization approaches.
Interactive Dashboards

The primary goal of Key Risk Indicators (KRIs) is to identify and quantify risks in real time, allowing for proactive measures to prevent trial disruptions and non-compliance. KRIs monitor critical metrics like screen failure rates, adverse events (AEs), serious adverse events (SAEs), and query rates. They act as early warning signals, enabling sponsors, CROs, and regulators to detect potential issues before they escalate, ensuring the highest standards of trial quality and patient safety.
Site Metrics

Monitor the performance and compliance of each clinical trial site to ensure data quality, patient safety, and operational efficiency. Our site metrics are essential for the effectiveness and timeliness of clinical monitoring activities, especially under risk-based monitoring frameworks.
Clean Patient Tracker (CPT)

With our CPT, ensure data readiness for analysis, accelerate database lock timelines, and help prioritize sites lagging in queries and source data verification (SDV) issues.
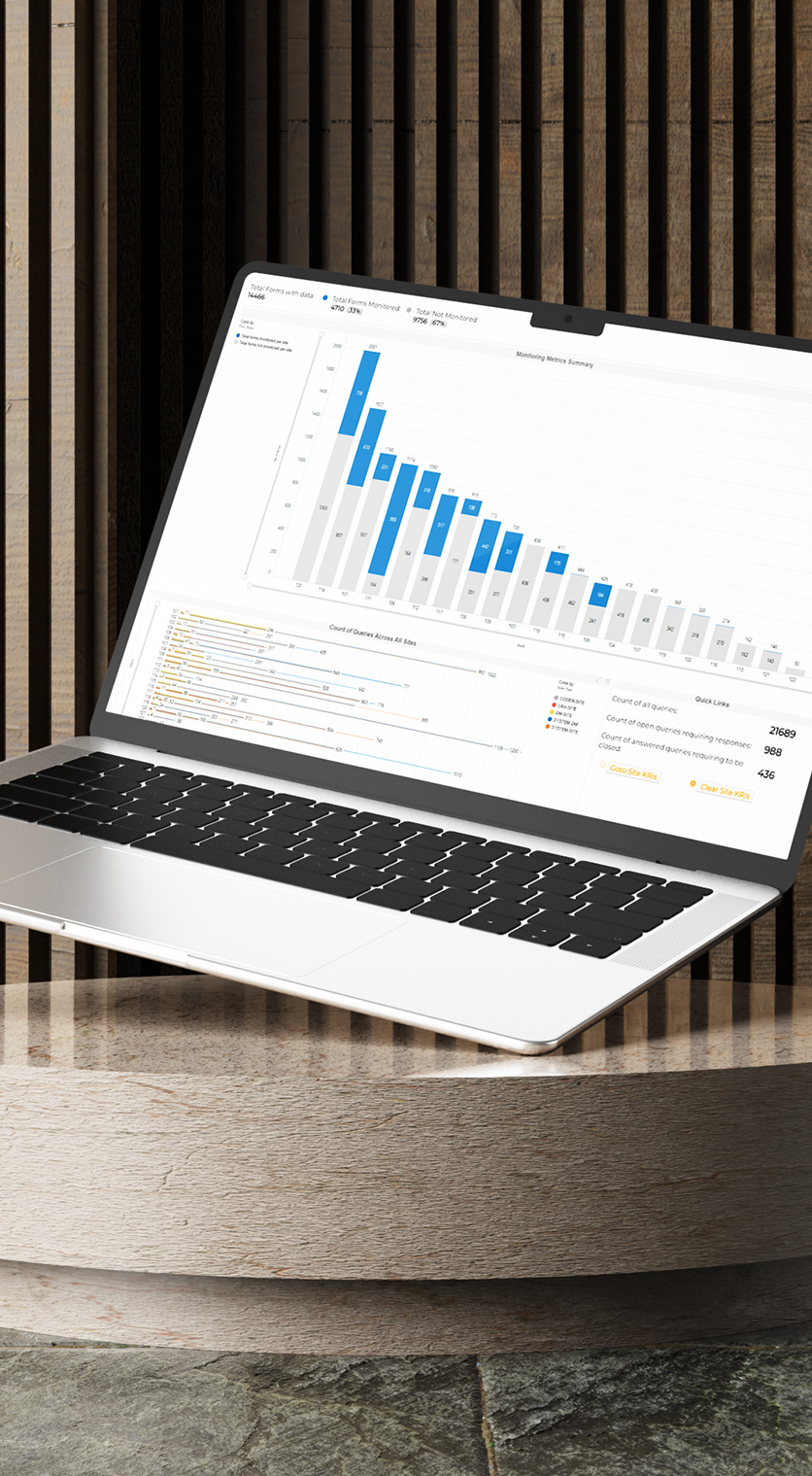
Spend less time understanding the issues and more time developing solutions.
Our Data Aggregation and Visualization services can be used separately or combined under our flexible FSP model, backed by:
- Skilled resources trained on your processes
- Therapeutic area-based teams with centralized governance
- Scalable capacity management for quick ramp-up and down
- Proven end-to-end process from data capture through submission-ready deliverables

Discover More
Related Services at Veranex
Clinical Data Management
Clinical Data Management
Our clinical data management will transform your trial data into actionable insights, ensuring unparalleled accuracy, efficiency, and compliance that drive successful outcomes.

Regulatory Consulting
Regulatory Consulting
Accelerate Your Regulatory Submission, partner with our regulatory experts to transform your biostatistics into FDA-ready documentation

Biostatistics & Statistical Programming
Biostatistics & Statistical Programming
Our Biostatistics and Statistical Programming turns complex clinical data into actionable insights, ensuring your research drives impactful decisions and successful outcomes.

Study Monitoring
Study Monitoring
Veranex delivers expert monitoring services that identify risks early, optimize site performance, and keep your studies on track for successful regulatory submissions.

Contact us today to enhance your decision-making and drive impactful results!
Unlock the potential of your clinical trials with Veranex's intuitive data visualizations and Key Risk Indicator dashboards. Contact us today to see how we can enhance your decision-making and drive impactful results!