2 min read
Avoid These Four Medical Device Supply Chain Woes
Anyone who works in medtech product development knows that surprises are a guarantee. That’s why it’s important to create stability and...
Traditional CROs fragment device development with costly hand-offs and learning curves. Veranex unites the essential disciplines for medical device & diagnostic development under one roof from sketch to evidence-generation to market launch.
All connected. All aligned. All accelerating your path to market—delivering breakthrough devices and diagnostics that improve patient lives sooner.
Breakthrough innovation requires more than great solutions; it demands deep expertise and insight. Veranex packages outcome-driven solutions with 25+ years of specialized knowledge across major medtech categories, delivering integrated capabilities that solve your most pressing challenges faster and with greater certainty.
Purpose-built solutions. Proven results. User & Patient-centered innovation.
Whether you're transforming patient care or disrupting entire therapeutic categories, innovation requires more than great science, it demands velocity. Veranex was founded to bridge the gap between visionary concepts and market reality, combining proven expertise with agile execution to accelerate the innovations that matter most.
We are the Innovation CRO.
Legacy of excellence. Proven execution. Patient impact accelerated.
6 min read
Veranex : Aug 4, 2025 3:02:51 PM
Behind many successful medical devices are design and engineering firms that provide the technical expertise, regulatory knowledge, and development capabilities necessary to bring innovative healthcare solutions to market.
As products become more complex and regulations grow more stringent, especially as software and AI enter the medical device domain, selecting the right medical device design partner has become increasingly important. In addition to navigating complex and dynamic regulations, these firms must understand clinical workflows and environments. They should maintain deep knowledge of biocompatibility requirements, sterilization processes, quality management systems, designing for manufacturability, manufacturing transfer and manufacturing at scale.
Regulatory expertise is fundamental, with top firms maintaining teams of professionals, many of whom were previously employees of regulatory bodies, who specialize in device classification, risk management, clinical evaluation, global regulatory guidelines and changes, and regulatory submission processes. Top medical device design and engineering firms invest heavily in maintaining up-to-date knowledge of FDA regulations, ISO standards, and international compliance requirements across multiple classifications and global locations. This expertise is invaluable for avoiding costly delays and ensuring successful regulatory submissions.
Broad therapeutic area experience is another distinguishing factor. Leading firms have successfully developed devices across multiple medical specialties, including cardiology, orthopedics, diagnostics, robotics, surgical instruments, and patient monitoring systems among other technologies. Diverse experiences enable them to apply best practices and innovative approaches across different applications.
Design and engineering excellence, excellence that one can validate, is table stakes as well. During detailed design and development phases, leading firms provide mechanical engineering, electrical engineering, software development, and systems integration services. They conduct extensive testing and validation, including biocompatibility studies, performance testing, and human factors validation. Astute medical device design and engineering firms and their clients do well to incorporate human factors engineering (HFE) expertise, including recent HFE regulatory guidance and requirements throughout the process inclusive of the earliest iterations or prototypes.
Manufacturing support has become increasingly important, with top firms offering everything from manufacturing feasibility consulting to high-speed prototyping and fast proof of concept services, supply chain management and controlled build support. This end-to-end capability ensures that devices can be manufactured at scale with consistent quality and regulatory compliance.
Most importantly, leading firms have evolved beyond traditional design services to offer comprehensive solutions that span the entire product lifecycle, enabling clients to consolidate vendors and streamline development processes. They understand that successful medical device development requires seamless integration of research, industrial design, human factors, engineering, regulatory strategy, and manufacturing expertise—all working in concert to transform innovative concepts into commercially successful products.

Selecting an appropriate medical device design partner requires careful evaluation of several critical factors:
The best partnerships are formed with firms that view themselves as true collaborators in the innovation process, bringing not just technical expertise but strategic guidance that increases the likelihood of commercial success, along with comprehensive concept to commercialization expertise that means you don’t have to stop to engage an external resource. They should also understand how your business or funding mechanisms work. If a regulatory submission unlocks follow-on funding, strategic sequencing of activities can increase the overall likelihood of success. Having knowledge required at any phase, for any insight, accelerates commercialization timelines, reducing cost and accelerating time to revenue.
In addition to navigating complex and dynamic regulations, these firms must understand clinical workflows and environments. They shouldmaintain deep knowledge of biocompatibility requirements, sterilization processes, and quality management systems.

Selecting an appropriate medical device design partner requires careful evaluation of several critical factors:
The best partnerships are formed with firms that view themselves as true collaborators in the innovation process, bringing not just technical expertise but strategic guidance that increases the likelihood of commercial success, along with comprehensive concept to commercialization expertise that means you don’t have to stop to engage an external resource. Having knowledge required at any phase, for any insight, accelerates commercialization timelines, reducing cost and accelerating time to revenue.

As the medical device landscape grows more complex, the critical question for innovators is no longer just "Can you design our device?" but "Can you assess every risk to ensure our product achieves regulatory approval and market success?"
An integrated partner like Veranex addresses this by strategically managing risks at every stage of development. This approach provides confidence to stakeholders and ensures the creation of a robust, reliable device poised for market success, ultimately transforming promising technologies into market-leading solutions.
2 min read
Anyone who works in medtech product development knows that surprises are a guarantee. That’s why it’s important to create stability and...
5 min read
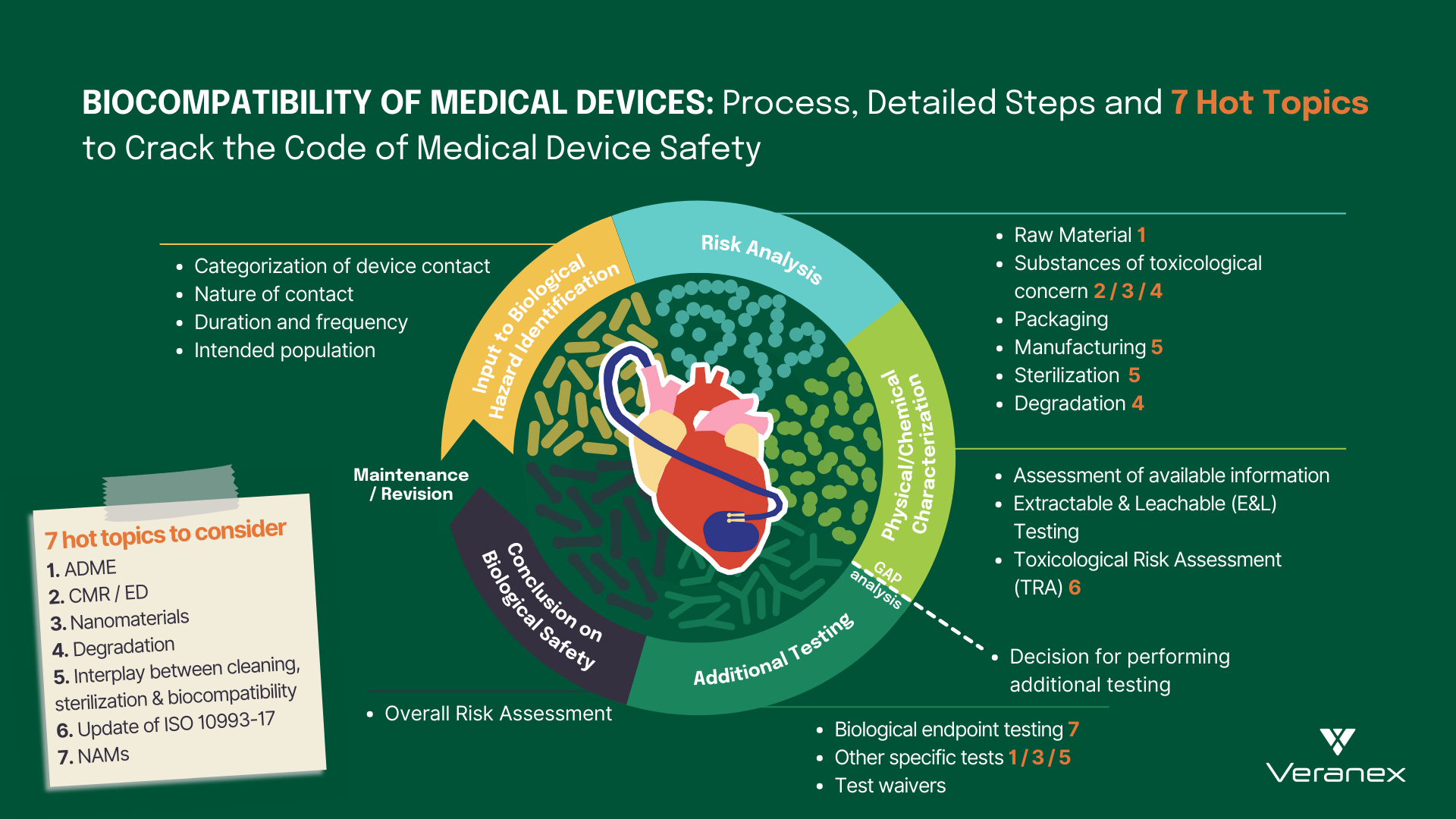
The landscape of modern healthcare has been completely transformed by the development of cutting-edge medical devices. From life-saving implants...

5 min read
How do regulatory requirements within the medical device industry impact a database build? What are the important items to be aware of? In this...