3 min read
How to Design an Efficient Preclinical Testing Study
A medical device’s pathway from concept to commercialization can be fraught with pitfalls, setbacks, and a need to recurring, significant...
Traditional CROs fragment device development with costly hand-offs and learning curves. Veranex unites the essential disciplines for medical device & diagnostic development under one roof from sketch to evidence-generation to market launch.
All connected. All aligned. All accelerating your path to market—delivering breakthrough devices and diagnostics that improve patient lives sooner.
Breakthrough innovation requires more than great solutions; it demands deep expertise and insight. Veranex packages outcome-driven solutions with 25+ years of specialized knowledge across major medtech categories, delivering integrated capabilities that solve your most pressing challenges faster and with greater certainty.
Purpose-built solutions. Proven results. User & Patient-centered innovation.
Whether you're transforming patient care or disrupting entire therapeutic categories, innovation requires more than great science, it demands velocity. Veranex was founded to bridge the gap between visionary concepts and market reality, combining proven expertise with agile execution to accelerate the innovations that matter most.
We are the Innovation CRO.
Legacy of excellence. Proven execution. Patient impact accelerated.
5 min read
Veranex : Sep 10, 2025 9:01:32 AM
Contributors: Nicolas Borenstein (DVM, MsC, PhD), Jennifer Gordon, Michael Sweet
When evaluating preclinical contract research organizations (CROs) for your medical device, choosing based on price alone is a false economy. The wrong decision can lead to study failures, regulatory delays, and costly rework setting your innovation months or even years. The real value lies in a partner with the scientific expertise, operational excellence, and strategic foresight to guide your innovation seamlessly from preclinical studies through commercialization.
It’s tempting to view preclinical contract research and testing as a commodity, like shopping for cars. After all, any car can technically get you from point A to point B. But would you choose the cheapest vehicle if reliability, safety, and performance were mission-critical? Of course not.
When your innovation is on the line, you need a partner who can guarantee not just the drive – but a product with the precision design, quality, and expert navigation needed to reach your destination without detours or breakdowns.
Preclinical testing is no different. Cutting corners on your CRO partner might save you upfront – but can cost far more in repeat studies, regulatory setbacks, or compromised study data.
Whether you're a clinician-innovator or engineer with a breakthrough concept or strong enhancement for a predicate device, a startup preparing for your first regulatory submission, or an established company like Medtronic, J&J or Stryker launching your next product line, the quality of your preclinical contract research partner directly impacts your path to market.

Credentialed Expertise That Delivers
The best preclinical CROs employ credentialed scientists and tenured professionals including biomedical engineers, board-certified veterinarians, regulatory experts, and GLP study specialists. This breadth of expertise ensures that every model, method, and milestone is backed by science, not guesswork. When you invest in top-tier expertise, you’re not paying for procedures, you’re securing peace of mind, strategic foresight, and accelerated success.
Model Development with Precision
Can your CRO develop relevant animal models for preclinical testing even when established predicates don't exist? If a predicate model doesn’t exist, we don’t force-fit a solution. We develop custom preclinical models. This ability to innovate and optimize preclinical models is a hallmark of excellence in preclinical contract research, especially crucial for truly groundbreaking devices.
Avoid the Learning Curve Tax
Choosing an inexperienced CRO means you’re funding their learning curve. Ensure you invest in a partner that comes prepared with deep therapeutic expertise, proven methodologies, and surgeon collaborations that reduce risk and increase confidence.
%20-%20Medium.jpg?width=1200&height=750&name=Illustrations_Bandeau_site%20web%20IMMR%20(10)%20-%20Medium.jpg)
Purpose-Built Excellence
Don't assume facility quality. Verify it. When evaluating preclinical contract research providers, the quality of their facilities can significantly impact data reliability and study outcomes. Purpose-built environments, with features like hospital-grade operating rooms, validated equipment, and robust backup systems, ensure that procedural integrity is maintained from start to finish.
Hospital-Grade Surgical Environments
The less distinguishable a preclinical OR is from a top-tier hospital surgical suite, the better. These elements replicate real-world surgical conditions, providing invaluable insights into how a device performs in clinical scenarios. This not only improves the fidelity of the data collected but also gives consulting surgeons a realistic setting to assess device handling, usability, and performance.
GLP Compliance, Not Certification
Think of GLP preclinical testing studies like a regulatory board exam – not a practices test. Pilot studies certainly serve a purpose in protocol refinement and early insights, but safety studies submitted to FDA must adhere to Good Laboratory Practice (GLP) regulations under 21 CFR Part 58. As to the European Union, obtaining CE mark is a long and strenuous process which the MDR regulation has made even more difficult in the past years.
It’s important to note: regulatory authorities do not certify preclinical CRO’s in GLP in the United States. On the other hand, in the EU, preclinical studies must comply with EOCD GLP which can only be claimed if studies have been performed in OECD GLP-accredited facilities. The Veranex preclinical lab in Paris is, indeed, OECD GLP-accredited.
Assess a CRO’s credibility by asking:
How many GLP-compliant studies have they successfully completed?
How many have been submitted to FDA or other agencies
What were the outcomes of their most recent regulatory inspections?
Quality Assurance You Can Trust
Look for a robust, independent Quality Assurance unit, well-defined Standard Operating Procedures (SOP’s), and Study Directors fluent in GLP regulations and device-specific regulatory pathways including 510(k), IDE and PMA Submissions.
AAALAC Accreditation
While not required by FDA for GLP or non-GLP preclinical studies, AAALAC accreditation demonstrates a serious commitment to ethical animal care and scientific rigor. Lack of accreditation can increase regulatory scrutiny, introduce delays, and even jeopardize funding from grant agencies that require it. Accredited facilities are posted on the AAALAC website.
Equipment and Validation
Does the CRO have the right equipment, and is it validated and available on your timeline? Equipment used in GLP studies must be calibrated and validated to comply with regulatory requirements.
In-House Histopathology
CRO’s with internal pathology teams provide seamless integration, faster turnaround, and higher-quality insights. The best preclinical pathologists understand their findings impact every downstream regulatory and commercial milestone.
The Importance of Audits
Leading preclinical CROs welcome sponsor audits – and you should conduct them as part of your final CRO selection process. Their transparency signals confidence in their facilities, quality systems, and regulatory acumen.

Scientific Fluency
Top-tier CROs specialize in key therapeutic areas such as cardiovascular, neurology, ophthalmology, and orthopedics. Their in-depth knowledge of relevant models, instruments, and regulatory requirements enable them to anticipate challenges – not just react to them - delivering faster timelines, cleaner data, and higher surgical success rates.
Senior-Level Insight
Experienced professionals ask the right questions, even the ones sponsor haven’t thought of yet. Their early input, particularly in study design and pathology, helps avoid costly missteps and supports stronger submissions.
Clear, Detailed Estimates
Insist on comprehensive project estimates with transparent costs. Ambiguity in budgeting often leads to mid-study surprises that jeopardize your financial and development plans.
Meticulous Execution
Attention to detail extends beyond the operating room. From coordinating site visits to managing intra-procedural issues and producing polished final reports – every detail matters. Make sure your KOL’s and consulting surgeons feel supported and respected; their engagement is vital to your study’s success.
Regulatory Track Record
Ask how many GLP studies the CRO has supported that led to regulatory submissions, with minimal or no questions or additional data requests. A track record of fewer than 50 successful device submissions may warrant closer scrutiny of their capabilities.
Preclinical studies - both GLP and non-GLP - are a major investment in your device's future. Investing in the right preclinical contract research services will accelerate your regulatory pathway. The wrong choice can delay it, increase your costs, and compromise data integrity.
Whether you're initiating your first safety study or recovering from a misstep, prioritize capability, compliance, and credibility over cost alone. Your timeline, your reputation, and your patients are counting on it.
At Veranex, our track record speaks for itself. We combine preclinical rigor and regulatory compliance with a holistic, integrated development approach spanning across all device development and commercialization stages. With testing facility labs in Atlanta and Paris, we have completed more than 150 GLP-compliant studies resulting in more than 100 regulatory approvals for medical devices worldwide. Integrated pathology services in Paris and Worcester have also supported most of these GLP studies, with the expertise of multiple board-certified veterinary pathologists in Paris, Atlanta and Worchester specializing in medical device technologies.

3 min read
A medical device’s pathway from concept to commercialization can be fraught with pitfalls, setbacks, and a need to recurring, significant...

2 min read
Medical device innovators need trustworthy CROs for preclinical studies to help speed the process from concept to commercialization. Below are a...
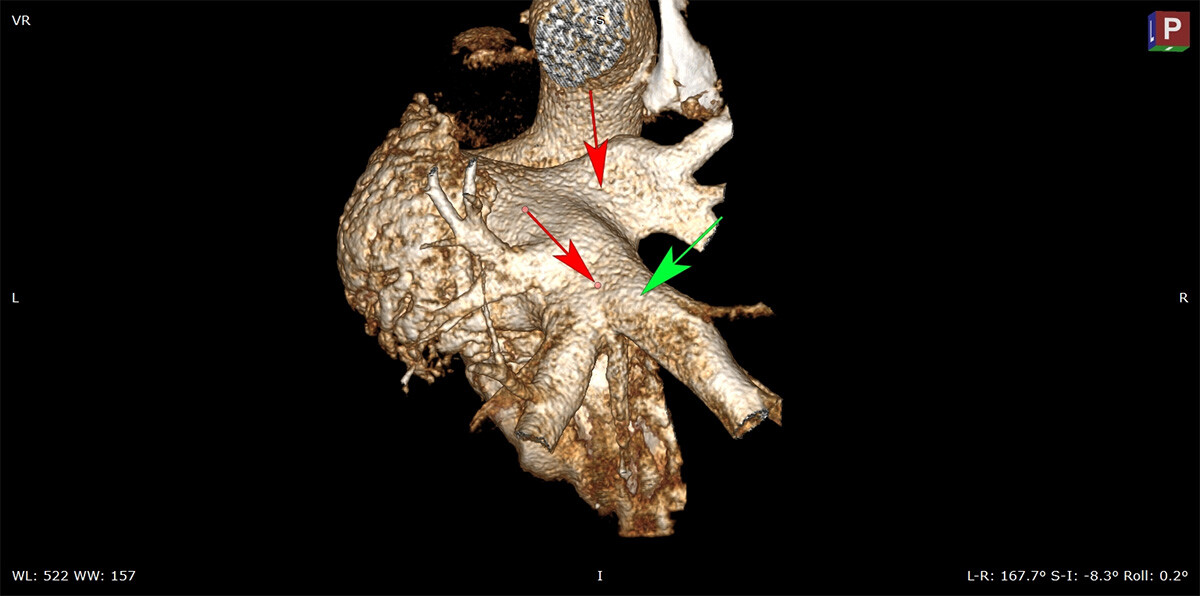
5 min read
Atrial fibrillation remains a leading cause of death and disability and the most common type of arrhythmia. While radiofrequency ablation and...