1 min read
Beyond the Procedure: Curiosity and Impact in MedTech
Some ventures launch on clever tech alone. Most of those fade. The ones that stick seem to orbit three ideas that came up again and again when Dr...
Traditional CROs fragment device development with costly hand-offs and learning curves. Veranex unites the essential disciplines for medical device & diagnostic development under one roof from sketch to evidence-generation to market launch.
All connected. All aligned. All accelerating your path to market—delivering breakthrough devices and diagnostics that improve patient lives sooner.
Breakthrough innovation requires more than great solutions; it demands deep expertise and insight. Veranex packages outcome-driven solutions with 25+ years of specialized knowledge across major medtech categories, delivering integrated capabilities that solve your most pressing challenges faster and with greater certainty.
Purpose-built solutions. Proven results. User & Patient-centered innovation.
Whether you're transforming patient care or disrupting entire therapeutic categories, innovation requires more than great science, it demands velocity. Veranex was founded to bridge the gap between visionary concepts and market reality, combining proven expertise with agile execution to accelerate the innovations that matter most.
We are the Innovation CRO.
Legacy of excellence. Proven execution. Patient impact accelerated.

1 min read
Some ventures launch on clever tech alone. Most of those fade. The ones that stick seem to orbit three ideas that came up again and again when Dr...

3 min read
Institutions and enterprises around the world are pushing the boundaries of medical innovation by applying advanced robotic technologies to address...

5 min read
How do regulatory requirements within the medical device industry impact a database build? What are the important items to be aware of? In this blog,...

4 min read
Risk-based quality management (RBQM) represents a core area of clinical development that systematically focuses on both safety and data quality risks...

2 min read
Exciting developments in the area of medical devices for the diagnosis, prognosis, treatment/management, and monitoring of Alzheimer’s disease (AD)...

3 min read
Our approach to Quality Management ensures that we’re always speaking the same language.

2 min read
On January 9, the United Kingdom (UK)’s Medicines and Healthcare Products Regulatory Agency (MHRA) released a regulatory framework – Roadmap towards...

4 min read
What is a rescue study? A rescue study is a clinical trial that is being conducted by a new vendor after having previously been conducted by another...

5 min read
What are some of the challenges with database builds for medical device studies, given the current regulatory requirements? What are the important...
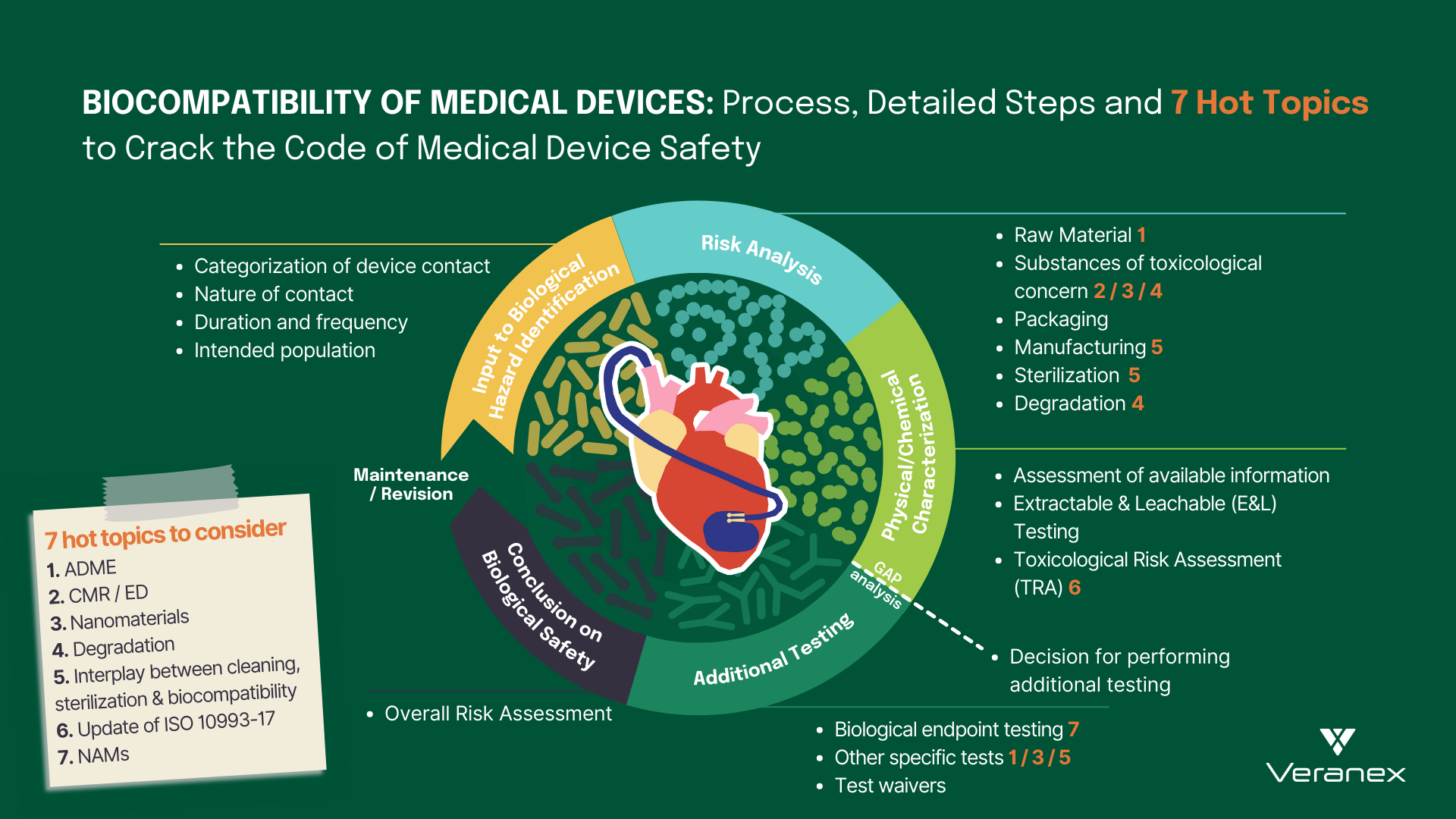
5 min read
The landscape of modern healthcare has been completely transformed by the development of cutting-edge medical devices. From life-saving implants to...