Veranex Blog
 Read More
Read More
 Read More
Read More
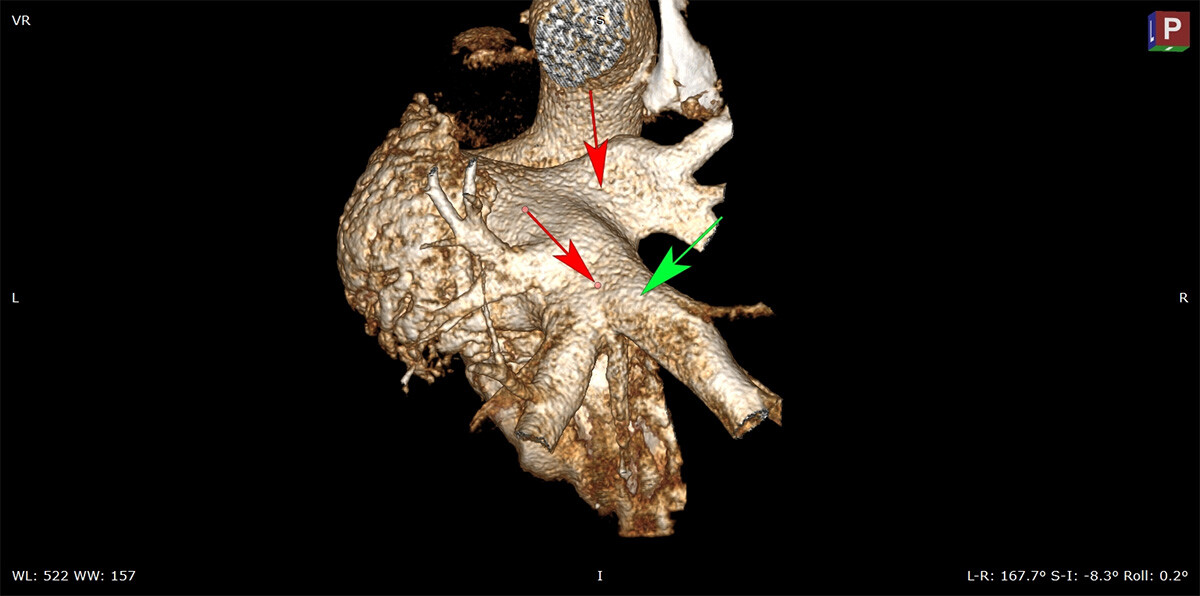
5 min read
The Promise of Pulsed Field Ablation and the Critical Role of Preclinical Science in its Advancement
Veranex: May 2, 2024 4:19:40 PM

1 min read
The Power of Generative Artificial Intelligence in Medical Writing
Yurii Kartashov: Apr 29, 2024 12:47:14 PM
1 min read
Harnessing AI Technology to Identify Adverse Events with Great Accuracy and Efficiency
Yurii Kartashov: Apr 29, 2024 12:46:35 PM
3 min read
Amending Regulation for MDR / IVDR: Key Takeaways for the Diagnostics Industry
Veranex: Apr 26, 2024 9:41:14 AM
 Read More
Read More

4 min read
2024 IVDR Sprint: 6 Reasons Why You Should Start a Performance Evaluation Early
Veranex: Apr 17, 2024 7:33:47 AM




