Veranex Blog
 Read More
Read More

4 min read
Optimizing PLM-OEM Collaborations: Key Strategies for a Smooth IVDR Transition
Veranex: Dec 12, 2024 9:12:06 AM
 Read More
Read More
 Read More
Read More

3 min read
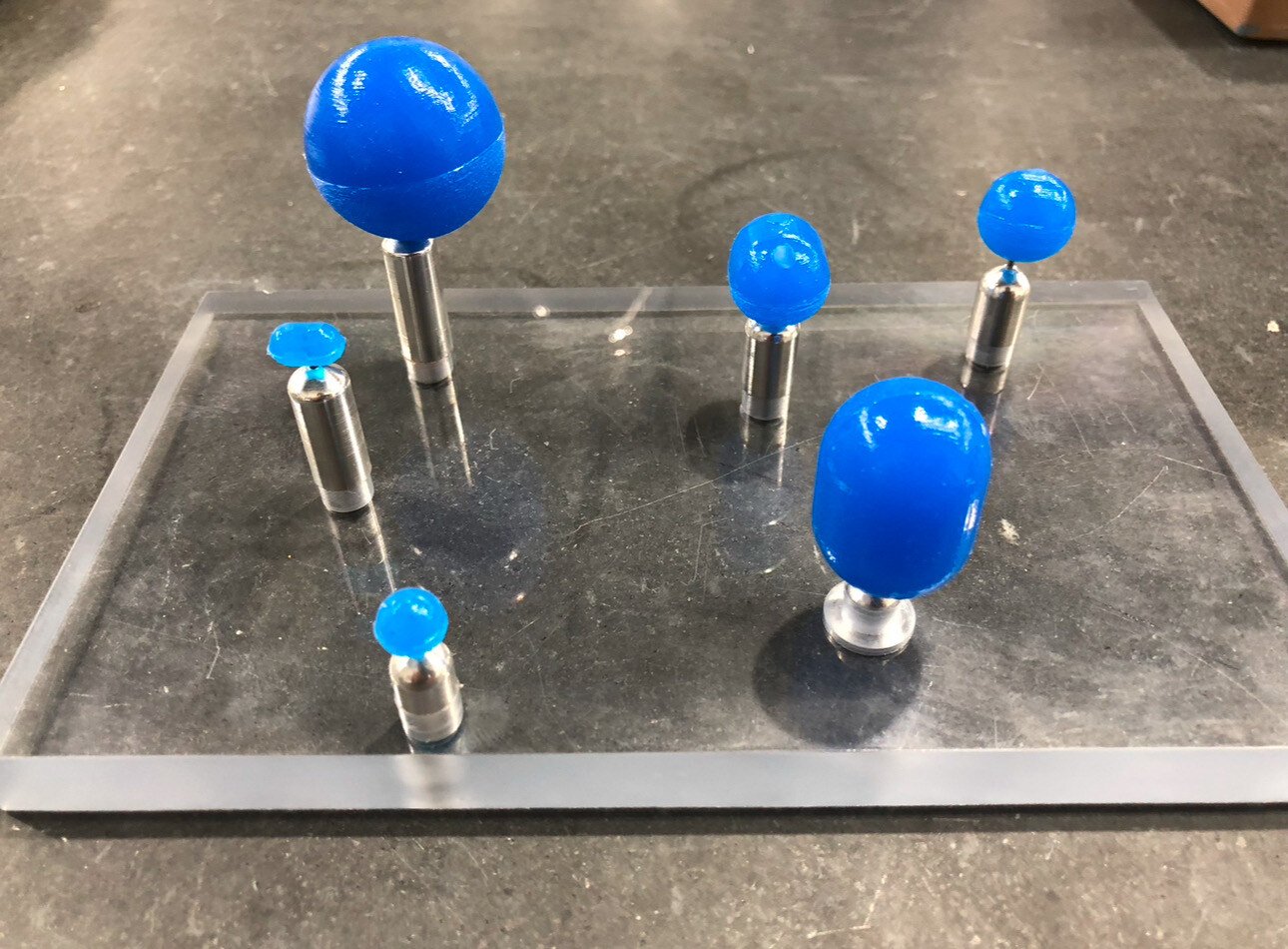
How 3D Printing Can Accelerate Your Product Development Timeline
Veranex: Nov 4, 2024 5:36:56 PM
5 min read
The Ins and Outs of Using ECMO and VAD in Preclinical Studies
Veranex: Nov 1, 2024 4:00:13 PM




-1.png?width=1114&height=672&name=Screenshot-2024-12-09-at-2.26.40%20PM%20(1)-1.png)
