GLP Preclinical Excellence: We Set the Standard in Medical Device Studies
We execute flawless GLP studies across every device category. State-of-the-art labs, innovative methodologies, and real-time analysis deliver the precision your innovation demands.
GLP Excellence That Regulators Trust
Exceptional capability in delivering regulatory-grade preclinical studies means zero FDA follow-up questions and accelerated market access for your breakthrough device. Our 95 % GLP success rate eliminates costly delays and regulatory risk, transforming complex studies into confident submissions that satisfy regulators every time.
GLP Results That Set the Standard
Our exceptional capability in delivering regulatory-grade preclinical studies transforms complex regulatory challenges into confident market entries. When industry leaders need GLP excellence they can stake their device's future on, they choose our proven methodology that delivers first-time regulatory wins without compromise.

Study Success Rate - significantly reduces time, cost and animal usage

patients treated with validated devices
GLP Preclinical Services: Excellence for Regulatory Success
Accelerate your path to regulatory approval with Veranex's dual-certified GLP facilities and proven track record. Our veterinary-led team delivers meticulous, consistent results across OECD and FDA regulatory pathways, supporting devices that have treated over 1 million patients worldwide.
GLP Compliant Study Types
-
OECD GLP Certified Studies (European regulatory pathway)
-
CFR 21 Part 58 GLP Compliant Studies (FDA regulatory pathway)
-
Chronic Safety and Efficacy Studies
-
Biocompatibility Testing
-
Device Performance Validation
Animal Models Available
- Large Animal Models: Swine (farm and minipig), Ovine, Bovine, Caprine
- Small Animal Models: Mice, Rats, Rabbits, Guinea Pigs
- Disease Models: Heart failure, acute MI, vascular pathology, bone defects
- Specialized Models: Pregnant ewes for fetal interventions

Comprehensive GLP Support Services
- Protocol Development and Regulatory Consultation
- In-House Gross and Histopathology Evaluation
- Medical Writing and Final Study Reports
- Quality Assurance and Data Management
- Regulatory Submission Support
- Post-Study Pathology Consultation
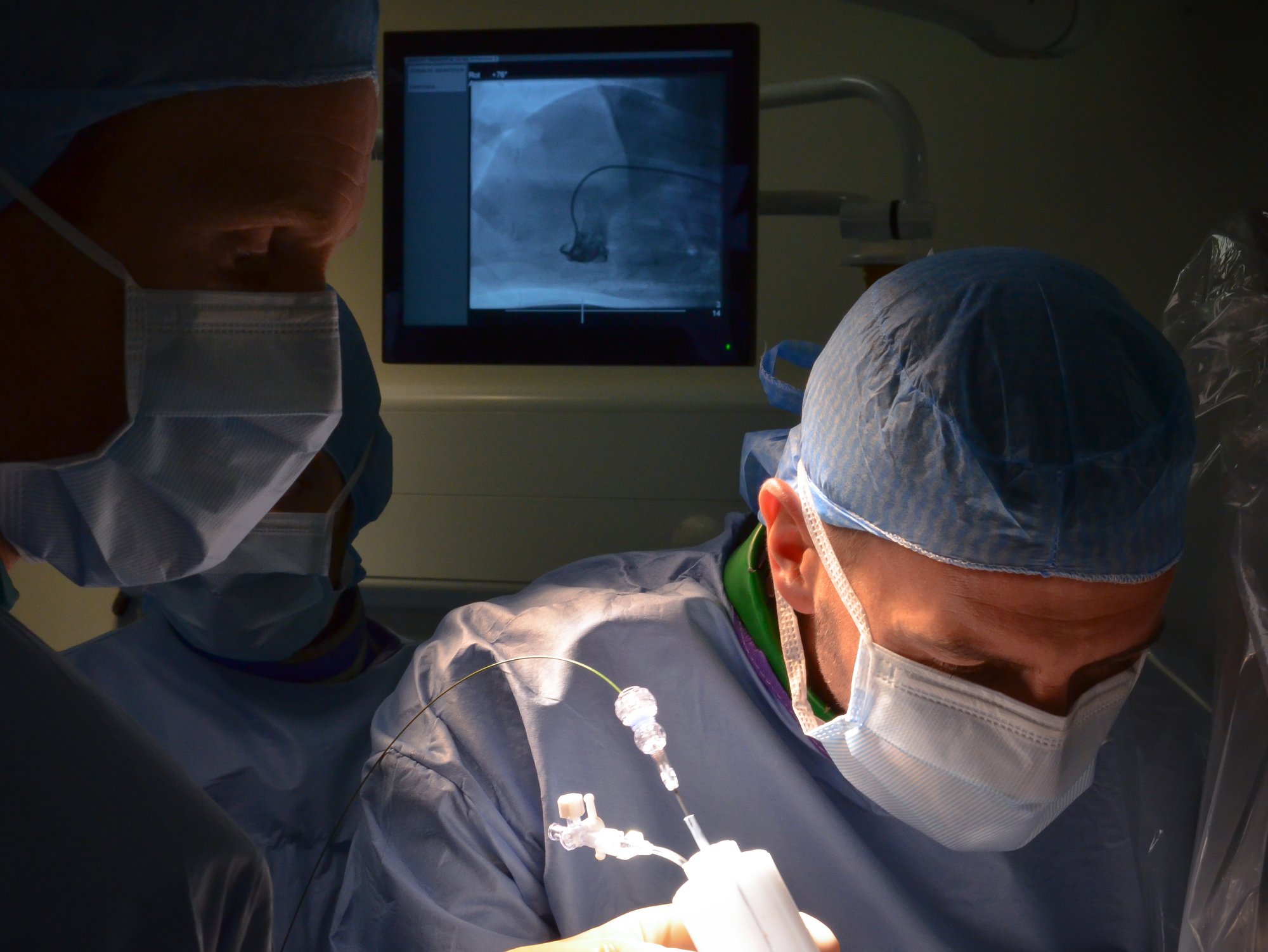
Advanced Imaging and Monitoring
- CT and MRI Imaging
- Echocardiography (TTE, TEE, ICE)
- 3D Fluoroscopy and IVUS/OCT
- Real-time Physiological Monitoring
Accreditations
- OECD GLP Certified
- CRF 21 Part 58 GLP Compliant
- FDA – registered and inspected
- Fully accredited by the French regulatory authorities
- USDA Registered
- DEA Registered
- OSHA compliant
- PHS Assurance
- AAALAC Accreditation




What Clients Say
"At the preclinical stage of medical device product development, there is an important opportunity to identify and implement significant improvements before first clinical use. Veranex provides extensive expertise and capabilities in the critical preclinical stage of medical device product development."
Senior Director of Product Development, Leading Medical Device Company
What Clients Say
"The facilities are top tier. We designed the facilities to make it easy to move from concept through protocol to budgeting and now it happens as efficiently as possible." More than a decade later, this cardiovascular surgeon continues to develop groundbreaking medical technologies from concept to cure to commercialization at Veranex."
Medtech Startup
What Clients Say
"We needed a partner capable of developing robust GLP protocols to ensure ethical research practices while meeting all safety and quality standards for regulatory approval. Equally important was the laboratory's execution capabilities—their understanding of training requirements and documentation protocols essential for our objectives. Our partnership with Veranex has been unwavering, and we have no plans to change that. The team's professionalism and attention to detail are exceptional, and we value their expertise so highly that we wish we could utilize their services for our European research programs as well."
CEO, Neurovascular Device Company
Our Preclinical Expertise
Leading GLP Expertise Across Critical Therapeutic Areas
Cardiovascular
Vascular Intervention
Neurosurgery and Neuromodulation
Targeted Drug Delivery

Gastroenterology and Urology
Orthopedics
Maternal-Fetal Health
Ophthalmology
Regenerative Medicine and Wound Healing

Meet the Team
Meet Our Leaders That Will Make Your GLP Study A Success
Nicolas Borenstein, DVM, MsC, PhD
Senior Vice President, Preclinical Services
Nicolas Borenstein serves as co-president of preclinical services, with extensive expertise in surgical and transcatheter preclinical science, particularly in cardiovascular and non-cardiovascular medical devices. A widely published author and peer-review journal reviewer, Nicolas combines academic excellence with hands-on surgical experience. He completed his veterinary medical and surgical training in Paris and Fort Collins, CO, earned his MSc in Surgical Science under Professor Alain Carpentier at Broussais Hospital, and received his PhD summa cum laude from University Paris Cité. His additional qualifications include specialized certifications in microsurgery, experimental surgery, and animal research from prestigious French institutions.
Luc Behr, DVM, MsC, PhD
Senior Vice President, Preclinical Services
Luc Behr serves as co-president of preclinical services, bringing more than 20 years of experience as a recognized thought leader in preclinical science. His deep expertise in surgical and transcatheter medical device design, combined with advanced medical imaging competencies, has helped hundreds of companies innovate sophisticated medical technologies and implant procedures. Luc's comprehensive understanding of the preclinical regulatory landscape complements his technical expertise. He completed his veterinary medicine degree and surgical residency at École nationale vétérinaire d'Alfort, followed by both his MSc and PhD in stem cell research from University Paris Cité, with additional training at The University of Missouri College of Veterinary Medicine.
Jeff White, DVM
Senior Vice President & Scientific Director, Preclinical Services
Jeff White serves as senior vice president and scientific director of preclinical services at Veranex, bringing extensive executive leadership experience in medical technology innovation and product development. Throughout his career, he has successfully led organizations through startups, mergers, acquisitions, and turnarounds, driving cumulative revenues exceeding $250M and contributing to over 70 FDA regulatory clearances and approvals. His expertise spans U.S. and international (GxP) regulations, strategic planning, and business development, with particular emphasis on building and managing high-performing teams across matrix-based academic, industry, and private equity-backed organizations. He holds a Doctor of Veterinary Medicine from Kansas State University and a Bachelor of Arts in Biology from The University of Kansas.
Sherry Farrugia
Senior Vice President, Preclinical Services
Sherry Farrugia serves as senior vice president of preclinical services at Veranex, bringing exceptional leadership experience in healthcare and life sciences innovation. Her distinguished career includes serving as CEO of T3 Labs and a decade at the Georgia Institute of Technology, where she led both the Global Center for Medical Innovation (GCMI) and the Pediatric Technology Center (PTC). Under her leadership, she has driven the commercialization of numerous medical devices, secured 90 funded projects, and generated multiple patents. A five-time Georgia Bio Award winner, including the prestigious Industry Growth Award, Sherry was named Women of the Year in Technology in 2018 and received the President's Award for Excellence in Multidisciplinary Team Research. She holds a BS in Chemistry and Physics from Auburn University and a certificate in global innovation from the University of Oxford.
Case Studies
Real World GLP Impact
Real GLP Results, Evidence-Driven Studies That Accelerate Regulatory Approval and Commercial Success

Advancing a First-of-Its-Kind Heart Failure Sensor with Veranex Preclinical Expertise
Situation
A leading medical device company developed the first implantable wireless sensor to monitor pulmonary artery pressure in heart failure patients. With over 1.4 million NYHA Class III heart failure patients in the U.S., the need for innovation was urgent. To meet stringent FDA Class III regulatory requirements, the company needed a preclinical partner with deep expertise and real-world clinical simulation capabilities.
Successes
- Veranex helped refine the sensor's design from an anchored right ventricular septum placement to a passively anchored pulmonary artery configuration, improving safety and ease of use.
- The team conducted GLP studies at Veranex to support their IDE submission, enabling first-in-human clinical trials.
- The collaboration resulted in a highly mature product at the time of clinical trial launch, reducing risk and accelerating regulatory approval.
- Following FDA approval in 2014, the device was acquired for $435 million.
Services by Veranex
- Implant design and delivery optimization
- Real-world cath lab simulation and testing
- Regulatory strategy and IDE support
- Procedural training development
- Long-term collaboration on next-gen device innovation

Turning a Preclinical Setback into Regulatory Success with Veranex
Situation
A medical device company's single-use radiofrequency applicator for intraoperative coagulation and ablation was on the path to FDA 510(k) clearance when early GLP preclinical studies raised red flags. A request for additional information from the FDA triggered company-wide concern, prompting a search for a preclinical partner capable of executing a flawless study under the highest compliance standards.
Successes
- Veranex executed a high-stakes GLP study that resulted in a 510(k) submission with zero follow-up questions and regulatory clearance in just 1.5 months.
- When anatomical model issues arose mid-study, Veranex swiftly enrolled replacements, avoiding delays and maintaining compliance.
- The team coordinated access to multiple surgical oncologists and board-certified veterinarians, ensuring FDA expectations for clinical exposure were met.
- Veranex's logistical precision, staff qualifications, and commitment to the project enabled the company to meet aggressive timelines and endpoints.
Services by Veranex
- GLP preclinical study design and execution
- Rapid model replacement and scheduling coordination
- Access to surgical specialists and veterinary staff
- Full-service preclinical logistics and compliance management

A Preclinical Partnership Driving Stroke Innovation
Situation
A neurovascular device company, led by a neurovascular neurosurgeon and experienced CEO, set out to improve outcomes in acute ischemic stroke by developing a new generation of stent retrievers. Their thrombectomy platform was designed to overcome limitations of legacy devices by capturing all clot types, including firm and organized thrombus, more effectively and with fewer passes.
Successes
- Since 2016, the company has partnered with Veranex on 7–10 GLP and non-GLP preclinical studies to develop and refine their thrombectomy platform.
- The studies directly supported the company's CE mark approval in 2017 and laid the groundwork for an expanded product line.
- Veranex provided rapid model replacement, expert coordination with surgical specialists, and full regulatory protocol support.
- The device demonstrated improved first-pass success and reduced clot fragmentation, even with calcified clots.
- The company credits Veranex's professionalism, regulatory acumen, and collaborative approach as critical to their development and commercialization success.
Services by Veranex
- GLP and non-GLP preclinical study execution
- Access to surgical specialists and board-certified veterinarians
- Onsite prototyping and early data collection
- Strategic guidance for CE mark and product line expansion
Ready to Accelerate Your Medtech Vision?
Transform your early-stage device concept into a clinical reality with our agile, expert-driven non-GLP preclinical research services.
Regulatory Services
Regulatory Consulting from Our Experts
Transform regulatory challenges into competitive advantages with our expert guidance from concept to market approval.

Preclinical Services
Full Spectrum of Preclinical Services
Accelerate your device development with our full spectrum of preclinical services beyond GLP studies.
Preclinical Facilities
Facilities to support your Innovation
Experience where innovation meets excellence in our FDA-inspected, dual-certified facilities spanning Atlanta, Worcester, and Paris.

Partner with GLP Excellence for Your Next Study
Connect with our veterinary-led team to explore how dual-certified GLP facilities and proven expertise can support your regulatory goals.










