Unmatched Preclinical Contract Research
Evidence based excellence, let our track record demonstrate the difference.
Our team of passionate experts combines extensive experience with precision and speed to drive your innovation forward.
Your Preclinical Success Fueled by Our Expertise
Proven preclinical contract research engineered to accelerate timelines

In-House Pathology Labs
Full Time Veterinarians

Years of Experience in Structural Heart and Cardiovascular

Submissions, no questions asked
What Clients Say
"Veranex have played a crucial role for us as we developed peripheral and neurovascular devices at our medical device companies. A decade after our first study we still go back for their interventional expertise in animal studies to support device development and regulatory approvals. Best of luck for the next 25 years!"
Chief Technology Officer, Leading Medical Device Company
What Clients Say
"I have been partnering with Veranex Preclinical Services for 15 years, and it has been an exceptional journey. Together, we've successfully transformed two groundbreaking concepts from napkin sketches into viable products in structural heart care."
CEO, Structural Heart Medical Device Company
What Clients Say
"Throughout the years, Veranex has played a crucial role in many of our accomplishments. Veranex consistently delivers excellence in both science and quality. Please continue the outstanding contribution to our industry for the next 25 years."
Co-founder and Chief Innovation Officer, Biomedical Technology Company
Therapeutic Areas of Expertise: Proven Success Across Medical Device Innovation
From structural heart breakthroughs to cutting-edge neuromodulation, our preclinical contract research team delivers specialized veterinary-led expertise across the full spectrum of medical device development. With over 3,000 procedures annually and devices treating 1+ million patients, our preclinical contract research provides the therapeutic depth and regulatory experience to accelerate your innovation from concept to clinical success.
Cardiovascular Experience:
- TAVR/SAVR - Pulmonary Stenting (2,500+ TAVI procedures completed)
- TMVR - TTVR - Chordal and Leaflet Repair (1,400 TMVR, 200 TTVR procedures)
- Ventricular Remodeling
- Left Atrial Appendage Closure
- Heart Failure Devices (CardioMEMS wireless sensor)
- Mechanical Circulatory Assist Technologies
- Cardiac Electrophysiology
- ECM Technology (160,000+ procedures across 975 hospitals)
Vascular Intervention Experience:
- Peripheral Arterial, Coronary, and Venous Stents
- AAA Stent Grafts
- Vascular Filters
- Surgical Sealants and Vascular Patches
- Drug Delivery
- Angioplasty Balloons
- Thrombectomy Devices (Vesalio NeVa™ platform)
- Monitoring Devices
- Embolization Devices (Ventor peripheral devices)
- Closure Devices
- Bioresorbable Scaffolds
- New Imaging Modalities
- Acute MI Models with Coronary Balloon Occlusion
Neurosurgery and Neuromodulation Experience:
- Cortical Stimulation
- Deep Brain Stimulation
- Peripheral Neuromodulation
- Nerve Ablation
- Neurovascular Stenting
- Clot Removal (Stroke intervention with improved first-pass success)
- Embolization Devices for AV Malformations
- Biological Pacemaker Gene Therapy
- Stroke and Traumatic Brain Injury Models

Targeted Drug Delivery Experience:
- Hydrogel-based Drug Delivery Systems (CorAmi AF treatment)
- Catheter-based Pericardial Space Delivery
- Cardiovascular Therapy (70% infarct size reduction achieved)
- Off-target Toxicity Reduction
- Novel Assay Development
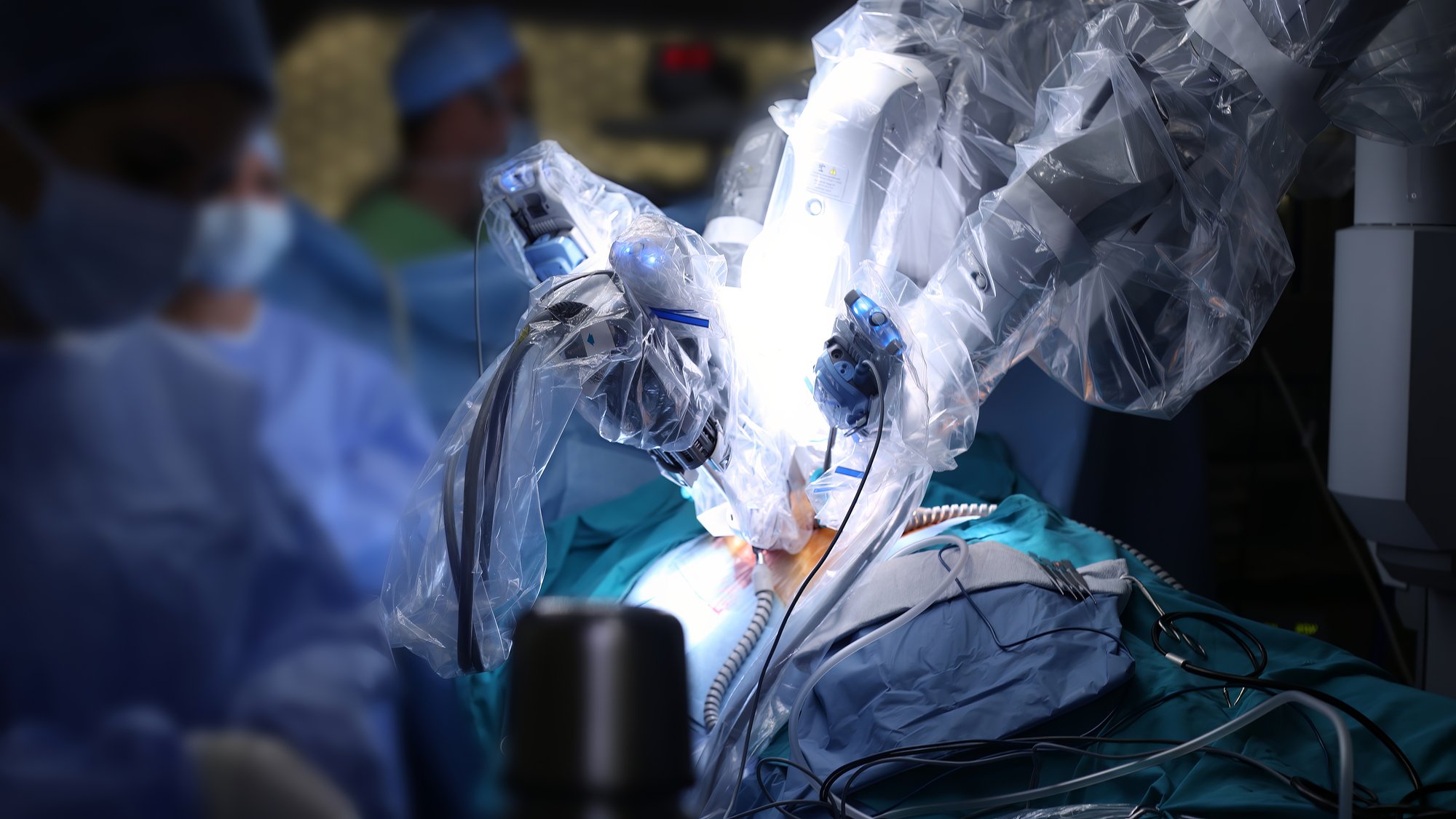
Gastroenterology and Urology Experience:
- Colon Resection and Defect Repair
- Bariatric Surgery
- Robotic Surgery
- Mesh Implantation
- Biopsy Devices
- Pancreatic and Liver Surgery
- Wound Healing
- Organ Laceration
- Stenting
- Interventional Radiology
- Embolic for Liver and Kidney
- Renal Denervation Models
- Renal Calculi and Stress Urinary Incontinence Models
- Acute Kidney Injury Models
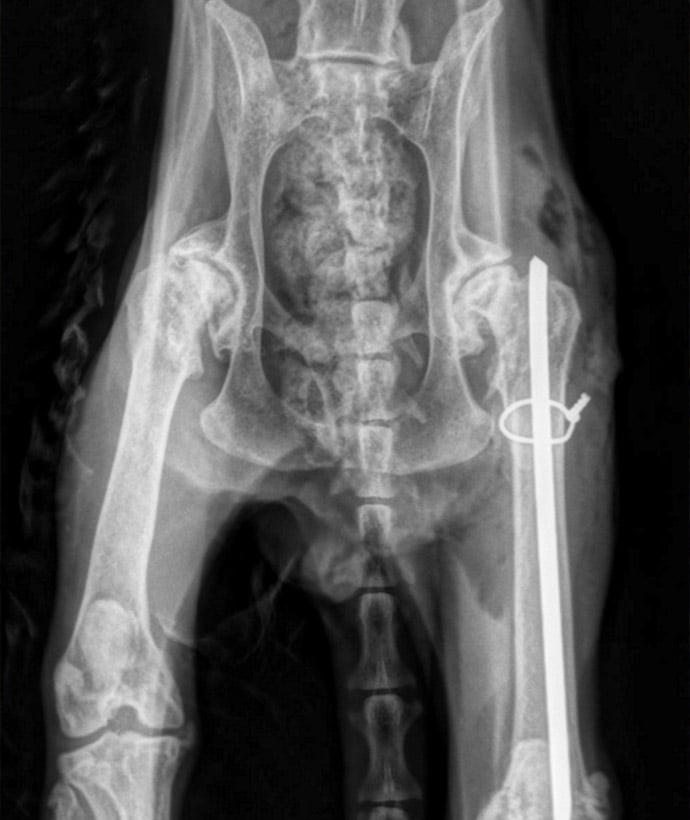
Orthopedics Experience:
- Tendon and Bone Repair
- Tendon Replacement
- Bone Segmentation and Fracture Healing
- Osseointegration
- Spine Fusion
- Infection
- Degenerative Disk Disease
- Prosthetics
- Amputation
- Osteochondral Defect
- Spinal Laminectomy Models
- Bone-Cartilage Defect Models

Maternal-Fetal Health Experience:
- Epidural Guidance Systems (Neuraline - reducing 1-in-8 complication rates)
- Spinal Needle Guidance (Ethos Medical improvements)
- Fetal Intervention Studies
- Women's Health Device Development
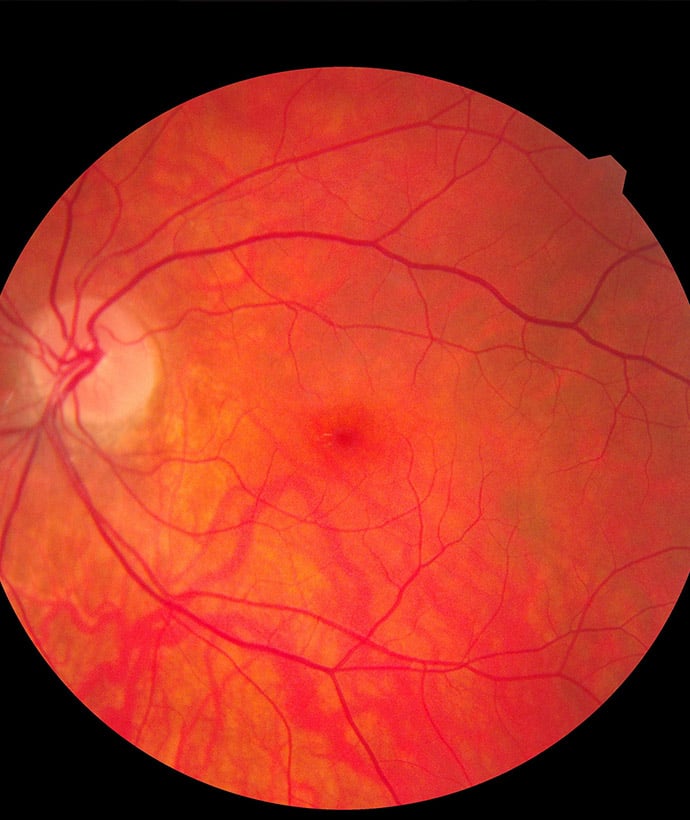
Ophthalmology Experience:
- Ocular Device Testing
- Specialized Animal Models for Eye Research

Regenerative Medicine and Wound Healing Experience:
- Cardiovascular Regeneration
- Myocardial Infarction Repair
- Extracellular Matrix Injections (CorMatrix ECM technology)
- Ablation Therapy
- Stem Cell Therapy
- Orthopedic Bone Regeneration
- Laminectomy
- Gene Therapy Applications
- Tissue Regeneration Studies

See Where Excellence Happens Explore our World Class Facilities
- Paris, France
- Atlanta, Georgia, USA
- Worcester, Massachusetts, USA
Preclinical Contract Research Across the Full Development Journey

From Ideation to Early Validation
- Initial proof of concept studies
- Product design iteration and troubleshooting
- Real-time problem-solving with early prototypes
- Working with nascent technologies
- Full assignment of any new IP to client

Available Animal Models
- Large Animal Models: Swine (Farm and Mini-pig: Yucatan/Gottingen), Bovine (Calves), Ovine (including Pregnant Ewes), Caprine, Canine (Small and Large dogs)
- Small Animal Models: Mice, Rats, Rabbits, Guinea Pigs
Human Cadavers: North America Only

Core Testing and Refinement
- Acute and chronic R&D/feasibility studies (Non-GLP)
- Consistency of surgical and technical team across cases
- Product design, delivery system, and placement procedure perfection
- Experience with multiple disease models
- Iterative design cycle: Design → Core Testing → Results → ReDesign

Disease Models Available
- Low EF heart failure/FMR/TR/FTR (coronary embolism)
- Acute MI (Coronary Balloon)
- DVT, arterial tortuosity, chronic total occlusions (CTO)
- Isolated beating-heart models
- Bone-cartilage defect models
- Acute Kidney Injury models
- Renal calculi and stress urinary incontinence models
- Stroke and traumatic brain injury models
- Renal denervation models
- Intestine-colon defect and inflammation models

Frozen Design and Compliance
- GLP-compliant studies (CFR 21 Part 58 & OECD)
- US FDA and European regulatory pathway support
- All procedures performed meticulously by veterinary surgeons
- 95% study success rate
- Dedicated Scientific and Quality Assurance teams

Advanced Equipment & Capabilities
- Imaging: CT Scanner, MRI, 3D Fluoroscopy, Echocardiography (TTE, TEE, ICE), IVUS/OCT
- Surgical: 10 Hybrid and Conventional OR suites, Cardiopulmonary Bypass
- Monitoring: PowerLab/MAC lab systems, Intellivue Monitors, LabChart
- Specialized: Endoscopy Towers, Laparoscopic Towers, Fiberscopes
- Real-time Streaming: V-Link platform for remote observation and recording
Discover the iCRO: The Innovation Contract Research Organization
Quality excellence begins in the design phase, not after your device is built. Traditional siloed approaches create costly iterations between product development, quality validation, regulatory approval, and clinical execution. Our integrated team ensures quality-first design accelerates regulatory strategy, clinical performance, and commercial potential from day one.
Meet the Team
Preclinical Excellence - Trusting Partnership, When our Team becomes Your Team.
Our 130+ preclinical contract research professionals across North America and Europe with extensive surgical expertise and unwavering dedication to your success. From 20 full-time veterinary surgeons to specialized pathologists and study directors, our world-class team delivers the complex evidence that regulatory bodies, investors, and clinical champions demand—safely, efficiently, and ethically.
Nicolas Borenstein, DVM, MsC, PhD
Senior Vice President, Preclinical Services
Nicolas Borenstein serves as co-president of preclinical services, with extensive expertise in surgical and transcatheter preclinical science, particularly in cardiovascular and non-cardiovascular medical devices. A widely published author and peer-review journal reviewer, Nicolas combines academic excellence with hands-on surgical experience. He completed his veterinary medical and surgical training in Paris and Fort Collins, CO, earned his MSc in Surgical Science under Professor Alain Carpentier at Broussais Hospital, and received his PhD summa cum laude from University Paris Cité. His additional qualifications include specialized certifications in microsurgery, experimental surgery, and animal research from prestigious French institutions.
Luc Behr, DVM, MsC, PhD
Senior Vice President, Preclinical Services
Luc Behr serves as co-president of preclinical services, bringing more than 20 years of experience as a recognized thought leader in preclinical science. His deep expertise in surgical and transcatheter medical device design, combined with advanced medical imaging competencies, has helped hundreds of companies innovate sophisticated medical technologies and implant procedures. Luc's comprehensive understanding of the preclinical regulatory landscape complements his technical expertise. He completed his veterinary medicine degree and surgical residency at École nationale vétérinaire d'Alfort, followed by both his MSc and PhD in stem cell research from University Paris Cité, with additional training at The University of Missouri College of Veterinary Medicine.
Jeff White, DVM
Senior Vice President & Scientific Director, Preclinical Services
Jeff White serves as senior vice president and scientific director of preclinical services at Veranex, bringing extensive executive leadership experience in medical technology innovation and product development. Throughout his career, he has successfully led organizations through startups, mergers, acquisitions, and turnarounds, driving cumulative revenues exceeding $250M and contributing to over 70 FDA regulatory clearances and approvals. His expertise spans U.S. and international (GxP) regulations, strategic planning, and business development, with particular emphasis on building and managing high-performing teams across matrix-based academic, industry, and private equity-backed organizations. He holds a Doctor of Veterinary Medicine from Kansas State University and a Bachelor of Arts in Biology from The University of Kansas.
Sherry Farrugia
Senior Vice President, Preclinical Services
Sherry Farrugia serves as senior vice president of preclinical services at Veranex, bringing exceptional leadership experience in healthcare and life sciences innovation. Her distinguished career includes serving as CEO of T3 Labs and a decade at the Georgia Institute of Technology, where she led both the Global Center for Medical Innovation (GCMI) and the Pediatric Technology Center (PTC). Under her leadership, she has driven the commercialization of numerous medical devices, secured 90 funded projects, and generated multiple patents. A five-time Georgia Bio Award winner, including the prestigious Industry Growth Award, Sherry was named Women of the Year in Technology in 2018 and received the President's Award for Excellence in Multidisciplinary Team Research. She holds a BS in Chemistry and Physics from Auburn University and a certificate in global innovation from the University of Oxford.
Case Studies
Real-World Preclinical Impact
See How Evidence-Backed Preclinical Contract Research Partnerships Accelerate Devices That Save Lives

Accelerating U.S. Regulatory Readiness for Respiratory Assist System with Veranex
Situation
A leading respiratory medical device company, developer of an innovative respiratory assist system, needed a preclinical research partner that could match their pace and budget as they transitioned from European success to U.S. FDA approval. Their early academic research environment proved too slow and rigid, prompting the search for a more agile and collaborative CRO.
Successes
- Veranex recommended transitioning from sheep to calf models, improving study manageability and physiological relevance.
- The collaboration enabled the client to refine protocols efficiently, particularly around anticoagulation—one of the most challenging aspects of device performance.
- Veranex's flexibility and scientific curiosity allowed the company to test new assays and techniques, fostering innovation and confidence ahead of GLP trials.
- The fourth study with Veranex served as a successful GLP dry run, setting the stage for regulatory submission.
- The client valued the long-term partnership and consistency developed with Veranex, prioritizing it over cost-based alternatives.
Services by Veranex
- GLP and non-GLP preclinical study execution
- Animal model consultation and optimization
- Protocol development and refinement
- Anticoagulation strategy testing
- Laboratory support for novel assay development
- Regulatory pathway alignment and GLP readiness

Veranex Sets Gold Standard in Preclinical Testing: Revolutionizing Drug Delivery for Atrial Fibrillation
Situation
Atrial fibrillation (AF) affects an estimated 10 million Americans, yet current treatments are either invasive or carry toxic side effects. A leading cardiac therapeutics company, led by a Chief Scientific Officer and Assistant Professor of Cardiology at a major university, is developing a minimally invasive hydrogel-based drug delivery system to treat AF more effectively. To bring this innovation to life, the company partnered with Veranex for preclinical testing and development.
Successes
- The company's catheter-based system delivers therapeutics directly to the pericardial space, reducing off-target toxicity and eliminating the need for open-heart surgery.
- Veranex provided a cath lab environment, fluoroscopy, and large animal expertise that enabled the client to test and refine its prototype efficiently.
- The Veranex team offered hands-on mentorship and scientific collaboration, helping the company navigate unfamiliar regulatory and technical processes.
- The partnership helped the client generate critical data and user feedback, positioning the company for FDA Pre-IND discussions and future clearance.
Services by Veranex
- Preclinical testing in cath lab and large animal models
- Prototype evaluation and procedural simulation
- Scientific mentorship and regulatory guidance
- Support for FDA documentation and study planning
- Collaboration with academic and clinical stakeholders

Biological Pacemakers: Revolutionary Gene Therapy Innovation Supported by Veranex
Situation
A leading pediatric cardiology researcher at a major university and children's hospital is developing a groundbreaking gene therapy to regenerate the heart's natural pacemaker cells—offering a potential alternative to implantable pacemakers, especially for pediatric patients. These children often face multiple high-risk surgeries due to the limitations of current pacemaker technology.
Successes
- The research team developed a gene therapy that converts heart muscle cells into pacemaker cells using a single gene delivered via mRNA.
- Preclinical studies at Veranex demonstrated that the therapy could restore and regulate heart rhythm in large animal models without implanted devices.
- Continuous ECG monitoring over 30 days showed near-normal heart rates and adaptive pacing, with no safety concerns identified by cardiologist review.
- The data generated at Veranex supported four major grants.
- Veranex's cardiac cath lab capabilities and electrophysiology expertise were instrumental in executing complex large-animal studies and meeting tight grant deadlines.
Services by Veranex
- Large-animal preclinical study design and execution
- Cardiac catheterization and electrophysiology testing
- 24/7 ECG data collection and analysis
- Grant support and documentation
- Translational research infrastructure and scientific collaboration
Accelerate Your Preclinical Success Today
Let's discuss how our proven preclinical expertise can fast-track your path to market.










