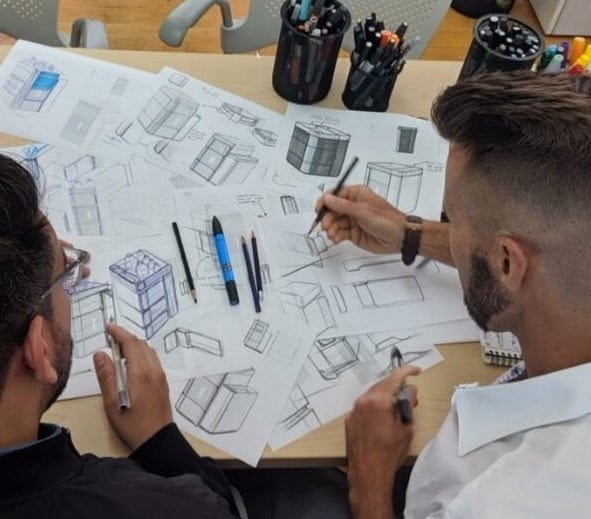
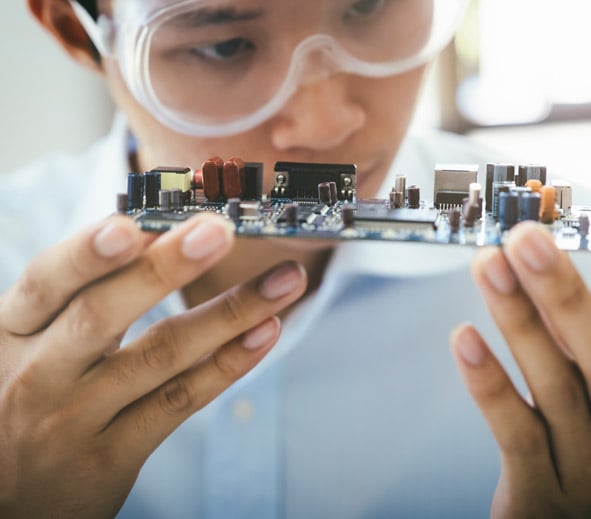
Drive Your Medical Device Development to Completion
Veranex delivers expert medical device development, guiding your innovation from initial concept to commercial reality.
Our integrated approach combines deep biomedical engineering services with agile processes, ensuring your product achieves market success and improves patient lives.
Proven Success in Medical Product Development
Comprehensive medical device development expertise, transforming innovative concepts into patient-ready solutions.

Over 35 Years of Refined Product Development Experience
In 2024 Our Teams Accomplished:

Hours gathering firsthand insights from our Research & Strategy team

Usability studies completed
Lines of new code

Builds executed to support submission testing
Forge Innovation with Strategic Biomedical Engineering Services
De-risk your development and have a clear path forward.
We translate foundational insights and user needs into robust design concepts and actionable strategies. Our teams conduct thorough research, define clear product specifications, and employ human-centered design principles to ensure your device is not only technically sound but also user-friendly and market-viable from the outset.
Engineer Excellence in Product & Software Development
Our expert engineers specialize in comprehensive medical product development, encompassing intricate mechanical design, electrical engineering, and sophisticated software architecture.
We develop everything from complex hardware to intuitive medical device user interfaces, ensuring robust performance and regulatory compliance. Agile methodologies and embedded quality assurance drive efficiency and reliability throughout development.
Assure Performance Through Rigorous Testing & Validation
Veranex ensures your medical device performs as intended, is safe for users and patients, and fulfills outlined needs crucial for market success.
Our development approach supports design refinement in parallel with test method development to ensure reliable data, de-risks verification, and builds a strong foundation for your regulatory submission. In parallel, our integrated teams execute usability assessments and formatives to lead to a successful validation of your device for submission.
Achieve Market Success with Seamless Manufacturing Integration
From pilot builds to supporting commercialization, we provide the expertise to scale efficiently and launch your product successfully.
We ensure your device is designed for manufacturability from the earliest stages. Our on-demand medical device contract manufacturing services offer flexible access to qualified facilities and robust supply chain experience with over 600 approved vendors.
Your Trusted Partner for Advanced Medtech Services
We are dedicated to your success in medical device development. We combine pioneering approaches with evidence-based practices to deliver solutions that are both innovative and reliable. Our patient-centric focus ensures that the end-benefit is always measured in lives improved. We work collaboratively, offering solution-oriented medical device consulting to accelerate your progress and navigate the complexities of the medtech landscape with clarity and confidence.

Innovate with Purpose
We leverage cutting-edge technologies and methodologies to deliver novel solutions.
Headline
Add your content here.

Execute with Precision
Our agile, data-driven processes ensure quality and speed to milestone.
Headline
Add your content here.
Focus on Patients
We design with the end-user in mind, prioritizing safety, usability, and improved outcomes.
Headline
Add your content here.

Deliver Reliable Results
Count on our experienced teams and robust quality systems for dependable development.
Headline
Add your content here.
Meet the Team
Meet Our Medical Device Design & Development Experts
Our dedicated teams of engineers, designers, researchers, and manufacturing specialists bring deep technical expertise and a collaborative spirit to every medical device development project. Their collective experience ensures your project benefits from innovative thinking and pragmatic execution, focused on achieving your strategic goals.
Tom Lutzow
VP, Research and Design
Meeting an unmet clinical need starts with exceptional design. That’s why Tom Lutzow and his team focus on human factors evaluation, engineering, and testing that drive safer, more effective, and commercially viable solutions. Their work blends user insights with technical excellence—grounded in regulatory foresight and manufacturability—to reduce risk and accelerate development. Tom has guided hundreds of innovators in shaping novel MedTech concepts into development-ready products. By embedding human-centered design into every phase—and collaborating closely with downstream functions like commercialization—his team ensures the right product gets to market, faster.
Joe Gordon
VP, Innovation and Technology
Based at our Providence, RI Innovation Center, Joe Gordon leads Veranex’s innovation engine—guiding early-stage concepts and generational enhancements into real-world solutions across more than 300 MedTech and in vitro diagnostic (IVD) projects. He specializes in translating ambiguity into actionable strategy, driving clarity through complexity, and aligning multidisciplinary teams to accelerate product realization. Whether it’s digital surgery platforms or wearable drug-delivery devices, Joe ensures each innovation is both breakthrough and buildable.
With over 20 years at the forefront of MedTech development, Joe plays a central role in full lifecycle programs—often leading from nascent idea through commercialization. He excels in navigating crowded patent landscapes and designing robust IP strategies that protect value creation. From capital systems to next-gen diagnostics, Joe’s diverse expertise and strategic foresight help set the innovation direction for clients aiming to reshape healthcare.
Jesse Gibson
Director, Software Development
As Director of Software Engineering, Jesse Gibson brings over 18 years of proven leadership in developing impactful healthcare and life-science products, specializing in complex embedded systems compliant with ISO 13485 and IEC 62304. His process-focused mindset and extensive experience successfully guiding multiple medical devices through the full design lifecycle, including verification, validation, and clinical trials, ensure your project benefits from rigorous quality, regulatory adherence, and on-time delivery. Jesse's passion for creating solutions that make a difference, coupled with his empathetic customer leadership, positions him to drive your software development initiatives to successful market launch.
Chris Vigneau
VP, Manufacturing Solutions
As Vice President of Manufacturing, Chris Vigneau leads the team providing integrated, end-to-end manufacturing solutions for your medical device project. With 20 years of operations leadership, his expertise in areas like operations management and strategic negotiation directly translates into high-quality, cost-effective, and compliant manufacturing processes for you. Chris ensures his team delivers the reliable, innovative support needed to navigate development challenges and position your device for successful commercialization.
Matt Perry
VP, Programs and Processes
As VP of Programs and Process, Matt Perry brings over 20 years of expertise in medical device development, with a proven track record guiding products from early customer research through to manufacturing and market release. His extensive background includes successfully leading the design, development, and manufacture of complex surgical devices through clinical studies and managing a diverse range of projects from high-volume disposables to electro-mechanical capital equipment. Matt's leadership ensures streamlined processes and successful program execution, delivering impactful medical device solutions for your business.
Tony DiBella
Sr Director, Product Dev. Program Mgmt
As a Senior Director of Product Development Program Management, Tony orchestrates Veranex’s diverse engineering teams across mechanical, electrical, firmware, software, and systems disciplines, ensuring cross-organizational excellence and consistency. With nearly two decades of medical device development experience, he is a proven leader who expertly guides multidisciplinary engineering teams through complex projects while serving as a trusted technical advisor and liaison to clients, all while adhering to stringent FDA 21 CFR 820 and ISO 13485 quality system requirements. Tony's leadership delivers cohesive and compliant product development outcomes for your critical medical device innovations.
Case Studies
Transforming Innovations into Life-Changing Realities
Explore how our integrated design and development services have helped clients overcome challenges, accelerate timelines, and bring impactful medical solutions to patients.

Guiding Investment in a Novel Imaging Solution
Situation
A client sought to validate and refine a hypothesis for a disruptive medical imaging solution, aiming to convert users of existing technologies by demonstrating superior performance, thereby de-risking development and guiding investment decisions.
Successes
- Strategic insights from 55 interviews within 4 months that confirmed the value proposition and guided critical go/no-go development and investment decisions.
- Identified granular, actionable user needs and desires across multiple clinical specialties, providing essential design inputs for product development.
- Economic and market analysis provided key insights into the adoption landscape and commercial models necessary for business success.
Services by Veranex
- Research & Strategy
- Commercial Strategy & Market Access
- Human-centered Industrial Design

Multi-System Design and Development
Situation
The client needed support envisioning and designing the next Endoscopic Cyclophotocoagulation (ECP) surgery system.
Successes
- Insights informed key design focus areas, driving the creation of 191 individual conceptual elements for 3 system concept directions then into down-selection
- Iterative testing, evaluation, and refinement of early prototypes resulted in generation of 2 volumetric system prototypes to refine the design and successfully integrated critical features, addressed key workflow challenges and mitigated use errors.
- The resulting design completed development and is now setting a new standard in the market for glaucoma treatment.
- Research and Strategy
- Human Factors
- UX / UI Design
- Design & Engineering
- Design Assurance

Pre-Mature Infant Humidification Device
Situation
The client needed Veranex to support pilot and initial commercial manufacturing of a pre-mature infant humidification device while they established a new manufacturing facility for long term production.
Successes
- 80 pilot units produced and distributed domestic and internationally
- Managed supply chain and commercial manufacturing for 1000+ units annually
- 4 years of interim commercial production support to a successful manufacturing transfer with seamless output.
Services by Veranex
- Manufacturing solutions
- Product Development
- Design Quality & Quality Assurance
- Supply Chain Management
- Project Management
Medical Device Development with The Innovation Contract Research Organization (iCRO)
With our breadth of services all collaborating in integrated teams, we ensure all aspects of your development project are covered with no additional vendors or handoffs for you.
Execute Your Medical Device Development Project with Confidence
Navigate the path from concept to commercialization with a trusted partner. Our comprehensive medical device development and medtech services are designed to de-risk your project, accelerate timelines, and achieve a successful market launch. Let us help you bring your innovative medical product development vision to life.